Page 40 - Strategies and Applications in Quantum Chemistry From Molecular Astrophysics to Molecular Engineer
P. 40
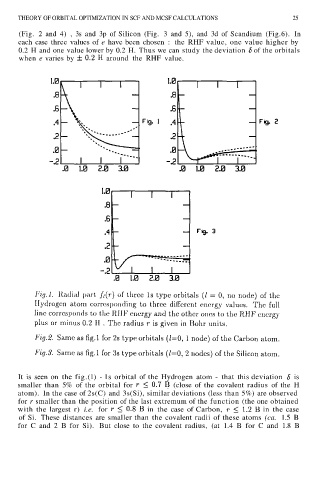
THEORY OF ORBITAL OPTIMIZATION IN SCF AND MCSF CALCULATIONS 25
(Fig. 2 and 4) , 3s and 3p of Silicon (Fig. 3 and 5), and 3d of Scandium (Fig.6). In
each case three values of e have been chosen : the RHF value, one value higher by
0.2 H and one value lower by 0.2 H. Thus we can study the deviation of the orbitals
when e varies by around the RHF value.
It is seen on the fig.(l) - 1s orbital of the Hydrogen atom - that this deviation is
smaller than 5% of the orbital for (close of the covalent radius of the H
atom). In the case of 2s(C) and 3s(Si), similar deviations (less than 5%) are observed
for r smaller than the position of the last extremum of the function (the one obtained
with the largest r) i.e. for in the case of Carbon, in the case
of Si. These distances are smaller than the covalent radii of these atoms (ca. 1.5 B
for C and 2 B for Si). But close to the covalent radius, (at 1.4 B for C and 1.8 B