Page 105 - Tandem Techniques
P. 105
Page 86
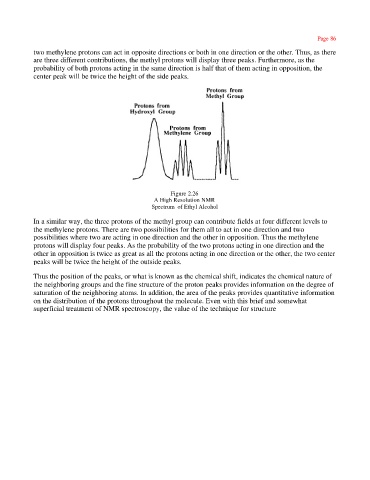
two methylene protons can act in opposite directions or both in one direction or the other. Thus, as there
are three different contributions, the methyl protons will display three peaks. Furthermore, as the
probability of both protons acting in the same direction is half that of them acting in opposition, the
center peak will be twice the height of the side peaks.
Figure 2.26
A High Resolution NMR
Spectrum of Ethyl Alcohol
In a similar way, the three protons of the methyl group can contribute fields at four different levels to
the methylene protons. There are two possibilities for them all to act in one direction and two
possibilities where two are acting in one direction and the other in opposition. Thus the methylene
protons will display four peaks. As the probability of the two protons acting in one direction and the
other in opposition is twice as great as all the protons acting in one direction or the other, the two center
peaks will be twice the height of the outside peaks.
Thus the position of the peaks, or what is known as the chemical shift, indicates the chemical nature of
the neighboring groups and the fine structure of the proton peaks provides information on the degree of
saturation of the neighboring atoms. In addition, the area of the peaks provides quantitative information
on the distribution of the protons throughout the molecule. Even with this brief and somewhat
superficial treatment of NMR spectroscopy, the value of the technique for structure