Page 28 - Teach Yourself Electricity and Electronics
P. 28
8 Basic physical concepts
Table 1-1. Continued
Element name Abbreviation Atomic number Atomic weight*
Tin Sn 50 120
Titanium Ti 22 48
Tungsten W 74 184
Unnilhexium** Unh 106 —
Unnilpentium** Unp 105 —
Unnilquadium** Unq 104 —
Uranium U 92 238
Vanadium V 23 51
Xenon Xe 54 132
Ytterbium Yb 70 174
Yttrium Y 39 89
Zinc Zn 30 64
Zirconium Zr 40 90
*Most common isotope. The sum of the number of protons and the number of neutrons in the nucleus. Most elements
have other isotopes with different atomic weights.
**These elements (atomic numbers 93 or larger) are not found in nature, but are human-made.
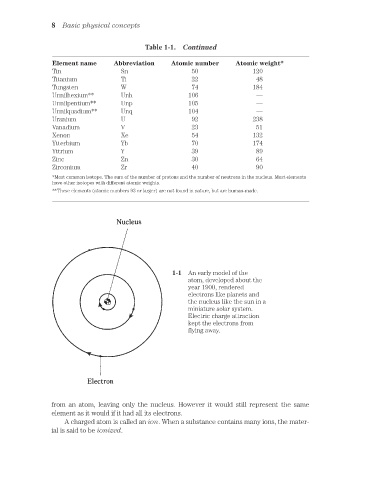
1-1 An early model of the
atom, developed about the
year 1900, rendered
electrons like planets and
the nucleus like the sun in a
miniature solar system.
Electric charge attraction
kept the electrons from
flying away.
from an atom, leaving only the nucleus. However it would still represent the same
element as it would if it had all its electrons.
A charged atom is called an ion. When a substance contains many ions, the mater-
ial is said to be ionized.