Page 115 - The Biochemistry of Inorganic Polyphosphates
P. 115
Char Count= 0
March 9, 2004
15:39
WU095/Kulaev
WU095-07
Participation in membrane transport 99
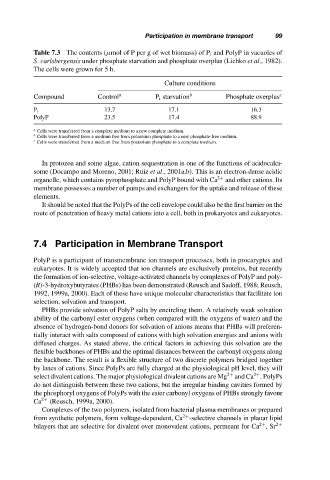
Table 7.3 The contents (µmol of P per g of wet biomass) of P i and PolyP in vacuoles of
S. carlsbergensis under phosphate starvation and phosphate overplus (Lichko et al., 1982).
The cells were grown for 5 h.
Culture conditions
Compound Control a P i starvation b Phosphate overplus c
13.7 17.1 16.3
P i
PolyP 23.5 17.4 88.9
a
Cells were transferred from a complete medium to a new complete medium.
b
Cells were transferred from a medium free from potassium phosphate to a new phosphate-free medium.
c
Cells were transferred from a medium free from potassium phosphate to a complete medium.
In protozoa and some algae, cation sequestration is one of the functions of acidocalci-
some (Docampo and Moreno, 2001; Ruiz et al., 2001a,b). This is an electron-dense acidic
organelle, which contains pyrophosphate and PolyP bound with Ca 2+ and other cations. Its
membrane possesses a number of pumps and exchangers for the uptake and release of these
elements.
It should be noted that the PolyPs of the cell envelope could also be the first barrier on the
route of penetration of heavy metal cations into a cell, both in prokaryotes and eukaryotes.
7.4 Participation in Membrane Transport
PolyP is a participant of transmembrane ion transport processes, both in procaryptes and
eukaryotes. It is widely accepted that ion channels are exclusively proteins, but recently
the formation of ion-selective, voltage-activated channels by complexes of PolyP and poly-
(R)-3-hydroxybutyrates (PHBs) has been demonstrated (Reusch and Sadoff, 1988; Reusch,
1992, 1999a, 2000). Each of these have unique molecular characteristics that facilitate ion
selection, solvation and transport.
PHBs provide solvation of PolyP salts by encircling them. A relatively weak solvation
ability of the carbonyl ester oxygens (when compared with the oxygens of water) and the
absence of hydrogen-bond donors for solvation of anions means that PHBs will preferen-
tially interact with salts composed of cations with high solvation energies and anions with
diffused charges. As stated above, the critical factors in achieving this solvation are the
flexible backbones of PHBs and the optimal distances between the carbonyl oxygens along
the backbone. The result is a flexible structure of two discrete polymers bridged together
by lanes of cations. Since PolyPs are fully charged at the physiological pH level, they will
select divalent cations. The major physiological divalent cations are Mg 2+ and Ca . PolyPs
2+
do not distinguish between these two cations, but the irregular binding cavities formed by
the phosphoryl oxygens of PolyPs with the ester carbonyl oxygens of PHBs strongly favour
Ca 2+ (Reusch, 1999a, 2000).
Complexes of the two polymers, isolated from bacterial plasma membranes or prepared
2+
from synthetic polymers, form voltage-dependent, Ca -selective channels in planar lipid
2+
bilayers that are selective for divalent over monovalent cations, permeant for Ca ,Sr 2+