Page 94 - The engineering of chemical reactions
P. 94
78 Reaction Rates, the Batch Reactor, and the Real World
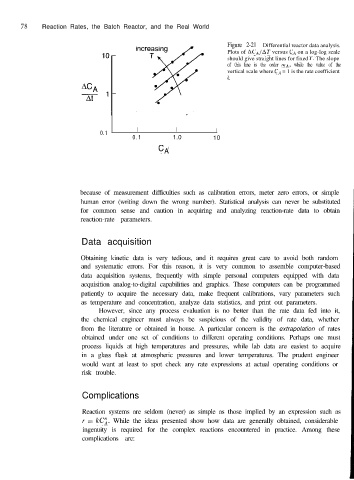
Figure 2-21 Differential reactor data analysis.
Plots of ACA/A7- versus CA on a log-log scale
should give straight lines for fixed 7’. The slope
of this line is the order rn~, while the value of the
vertical scale where CA = 1 is the rate coefficient
k.
I I I
0.1
0.1 1.0 10
CA
because of measurement difficulties such as calibration errors, meter zero errors, or simple
human error (writing down the wrong number). Statistical analysis can never be substituted
for common sense and caution in acquiring and analyzing reaction-rate data to obtain
reaction-rate parameters.
Data acquisition
Obtaining kinetic data is very tedious, and it requires great care to avoid both random
and systematic errors. For this reason, it is very common to assemble computer-based
data acquisition systems, frequently with simple personal computers equipped with data
acquisition analog-to-digital capabilities and graphics. These computers can be programmed
patiently to acquire the necessary data, make frequent calibrations, vary parameters such
as temperature and concentration, analyze data statistics, and print out parameters.
However, since any process evaluation is no better than the rate data fed into it,
the chemical engineer must always be suspicious of the validity of rate data, whether
from the literature or obtained in house. A particular concern is the extrapolation of rates
obtained under one set of conditions to different operating conditions. Perhaps one must
process liquids at high temperatures and pressures, while lab data are easiest to acquire
in a glass flask at atmospheric pressures and lower temperatures. The prudent engineer
would want at least to spot check any rate expressions at actual operating conditions or
risk trouble.
Complications
Reaction systems are seldom (never) as simple as those implied by an expression such as
r = kc:. While the ideas presented show how data are generally obtained, considerable
ingenuity is required for the complex reactions encountered in practice. Among these
complications are: