Page 71 - Thermal Hydraulics Aspects of Liquid Metal Cooled Nuclear Reactors
P. 71
46 Thermal Hydraulics Aspects of Liquid Metal Cooled Nuclear Reactors
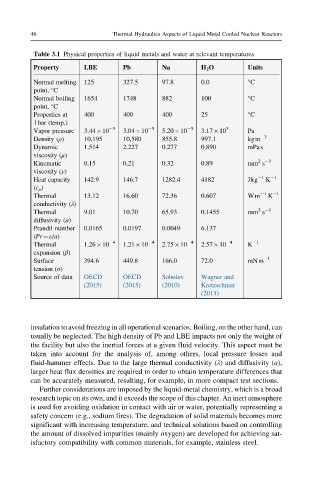
Table 3.1 Physical properties of liquid metals and water at relevant temperatures
Property LBE Pb Na H 2 O Units
Normal melting 125 327.5 97.8 0.0 °C
point, °C
Normal boiling 1654 1748 882 100 °C
point, °C
Properties at 400 400 400 25 °C
1bar (temp.)
Vapor pressure 3.44 10 5 3.04 10 5 5.20 10 5 3.17 10 3 Pa
Density (ρ) 10,195 10,580 855.8 997.1 kgm 3
Dynamic 1.514 2.227 0.277 0.890 mPas
viscosity (μ)
2 1
Kinematic 0.15 0.21 0.32 0.89 mm s
viscosity (ν)
Heat capacity 142.9 146.7 1282.4 4182 Jkg 1 K 1
(c p )
Thermal 13.12 16.60 72.36 0.607 Wm 1 K 1
conductivity (λ)
2 1
Thermal 9.01 10.70 65.93 0.1455 mm s
diffusivity (a)
Prandtl number 0.0165 0.0197 0.0049 6.137 –
(Pr¼ν/a)
Thermal 1.26 10 4 1.21 10 4 2.75 10 4 2.57 10 4 K 1
expansion (β)
Surface 394.6 449.8 166.0 72.0 mNm 1
tension (σ)
Source of data OECD OECD Sobolev Wagner and
(2015) (2015) (2010) Kretzschmar
(2013)
insulation to avoid freezing in all operational scenarios. Boiling, on the other hand, can
usually be neglected. The high density of Pb and LBE impacts not only the weight of
the facility but also the inertial forces at a given fluid velocity. This aspect must be
taken into account for the analysis of, among others, local pressure losses and
fluid-hammer effects. Due to the large thermal conductivity (λ) and diffusivity (a),
larger heat flux densities are required in order to obtain temperature differences that
can be accurately measured, resulting, for example, in more compact test sections.
Further considerations are imposed by the liquid-metal chemistry, which is a broad
research topic on its own, and it exceeds the scope of this chapter. An inert atmosphere
is used for avoiding oxidation in contact with air or water, potentially representing a
safety concern (e.g., sodium fires). The degradation of solid materials becomes more
significant with increasing temperature, and technical solutions based on controlling
the amount of dissolved impurities (mainly oxygen) are developed for achieving sat-
isfactory compatibility with common materials, for example, stainless steel.