Page 154 - Thermodynamics of Biochemical Reactions
P. 154
152 Chapter 8 Phase Equilibrium in Aqueous Systems
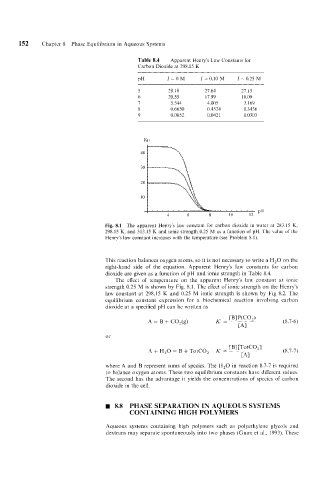
Table 8.4 Apparent Henry’s Law Constants for
Carbon Dioxide at 298.15 K
PH I=OM I = 0.10 M I = 0.25 M
5 28.18 27.64 21. I5
6 20.55 17.99 16.09
7 5.544 4.005 3.169
8 0.6650 0.4524 0.3456
9 0.0652 0.0421 0.0303
I
1
4 6 8 10 12 nH
r’’
Fig. 8.1 The apparent Henry’s law constant for carbon dioxide in water at 283.15 K,
298.15 K, and 313.15 K and ionic strength 0.25 M as a function of pH. The value of the
Henry’s law constant increases with the temperature (see Problem 8.1).
This reaction balances oxygen atoms, so it is not necessary to write a H,O on the
right-hand side of the equation. Apparent Henry’s law constants for carbon
dioxide are given as a function of pH and ionic strength in Table 8.4.
The effect of temperature on the apparent Henry’s law constant at ionic
strength 0.25 M is shown by Fig. 8.1. The effect of ionic strength on the Henry’s
law constant at 298.15 K and 0.25 M ionic strength is shown by Fig 8.2. The
equilibrium constant expression for a biochemical reaction involving carbon
dioxide at a specified pH can be written as
A = B + CO,(g) (8.7-6)
or
cBlCTotco,l
A + H,O = B + TotCO, K‘ = (8.7-7)
CAI
where A and B represent sums of species. The H,O in reaction 8.7-7 is required
to balance oxygen atoms. These two equilibrium constants have different values.
The second has the advantage it yields the concentrations of species of carbon
dioxide in the cell.
8.8 PHASE SEPARATION IN AQUEOUS SYSTEMS
CONTAINING HIGH POLYMERS
Aqueous systems containing high polymers such as polyethylene glycols and
dextrans may separate spontaneously into two phases (Guan et al., 1993). These