Page 358 - Thermodynamics of Biochemical Reactions
P. 358
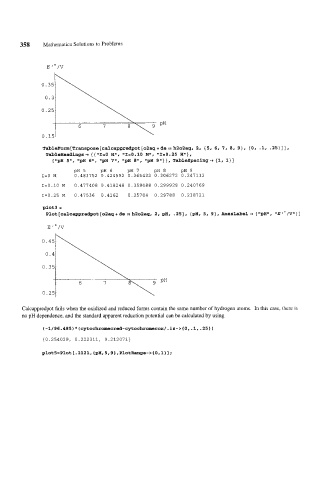
358 Mathematica Solutions to Problems
E '"/V
TableForm[Transpose[calcappredpot[o2aq+de == h202ag, 2, {5, 6, 7, 8, g}, {or .I, .25)]]
TableHeadings -f { { "I=O M", "I=O. 10 MI', "I=O. 25 M" } ,
{"gH 5", "pH 6", "gH 7", "gH 8", l1pH 9"}}, TableSgacing-t {l, I}]
PH 5 PH 6 PH 7 PH 8 PH 9
1=0 M 0.483752 0.424592 0.365432 0.306272 0.247112
I=O.lO M 0.477408 0.418248 0.359088 0.299928 0.240769
I=O.25 M 0.47536 0.4162 0.35704 0.29788 0.238721
plot3 =
=:
Plot [calcaggredgot [olaq + de h202ag, 2, gH, .25], {gH, 5, 9}, AxesLabel -t { tlpH1l, I /V" 1 I
"E
Calcappredpot fails when the oxidized and reduced forms contain the same number of hydrogen atoms. In this case, there is
no pH dependence, and the standard apparent reduction potential can be calculated by using
~-1/96.485~*(cytochromecred-cytochromecox/.is-~{0,.1,.25})
{0.254029, 0.222311, 0.212071)