Page 361 - Thermodynamics of Biochemical Reactions
P. 361
Redox Reactions 361
E '"/V
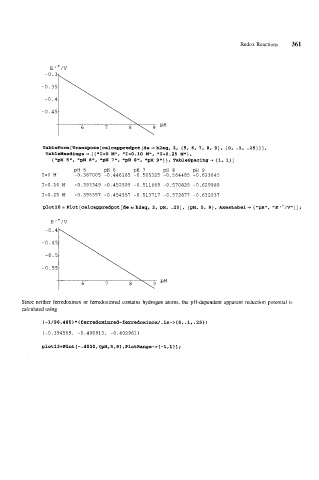
TableForm[Transpose[calcappredpot[de == h2aq, 2, (5, 6, 7, 8, 9), {0, -1, .25)]],
TableHeadings + { {"I=O MI1, "I=O.lO ME', "I=0.25 M"},
{"pH 5", "pH 6", "pH 7", "pH 8", "pH 9"}}, Tablespacing-, {I, l}]
PH 5 PH 6 PH 7 PH 8 PH 9
1=0 M -0.387005 -0.446165 -0.505325 -0.564485 -0.623645
I=O.10 M -0.393349 -0.452509 -0.511669 -0.570829 -0.629989
I=O.25 M -0.395397 -0.454557 -0.513717 -0.572877 -0.632037
E '"/V
-0.. 5.
-0.55-
6 7 8 \9 pH
Since neither ferredoxinox or ferredoxinred contains hydrogen atoms, the pH-dependent apparent reduction potential is
calculated using
~-1/96.485~*~ferredoxinred-ferredoxinox/.is-~{O,.l,~25})
I-0.394569, -0.400913, -0.402961}