Page 365 - Thermodynamics of Biochemical Reactions
P. 365
Redox Reactions 365
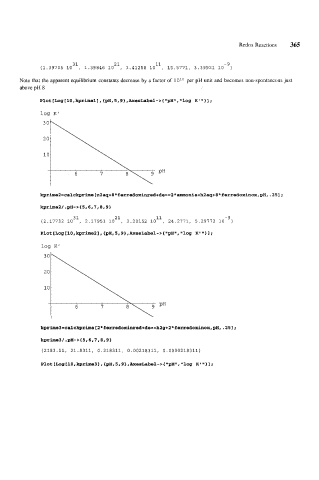
31 21 11 -9
{1.39705 10 , 1.39846 10 , 1.41258 10 , 15.5771, 3.39921 10 }
Note that the apparent equilibrium constants decrease by a factor of 10" per pH unit and becomes non-spontaneous just
above pH 8
Plot [Log [ 10, kprimel ] , {pH, 5,9 } , AxesLabel - > { "pH", log K ' 'I 1 1 ;
log K'
kprime2/.pH->{5,6,7,8,91
31 21 11 -9
{2.17732 10 , 2.17951 10 , 2.20152 10 , 24.2771, 5.29772 10 }
log K'
kprime3=calckprime[2*ferredoxinred+de==h2g+2*ferredoxinox,pH,.251;
kprime3/.pH->{5,6,7,8,9}
{2183.11, 21.8311, 0.218311, 0.00218311, 0.0000218311)
Plot [Log 110, kprime3 1, {pH, 5,9}, AxesLabel-> { "pH", "log K' 'I 1 I ;