Page 364 - Thermodynamics of Biochemical Reactions
P. 364
364 Mathematica Solutions to Problems
I -------
-0.75
6 7 8 9
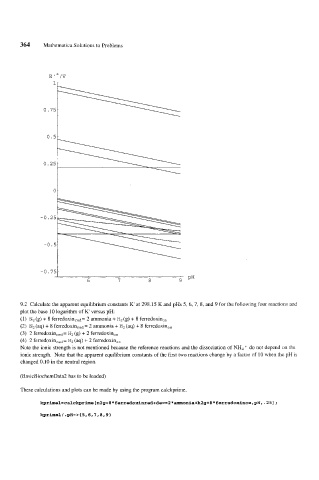
9.2 Calculate the apparent equilibrium constants K' at 298.15 K and pHs 5, 6, 7, 8, and 9 for the following four reactions and
plot the base 10 logarithm of K' versus pH:
(1) N2 (8) + 8 ferredoxinred= 2 ammonia + H2 (g) + 8 ferredoxin,,
(2) N2 (aq) + 8 ferredoxinred= 2 ammonia + H2 (aq) + 8 ferredoxin,,
(3) 2 ferredoxinred= H2 (8) + 2 ferredoxin,,
(4) 2 ferredoxinred= H2 (aq) + 2 ferredoxin,,
Note the ionic strength is not mentioned because the reference reactions and the dissociation of NH4 + do not depend on the
ionic strength. Note that the apparent equilibrium constants of the first two reactions change by a factor of 10 when the pH is
changed 0.10 in the neutral region.
(BasicBiochemData2 has to be loaded)
These calculations and plots can be made by using the program calckprime.