Page 369 - Thermodynamics of Biochemical Reactions
P. 369
Redox Reactions 369
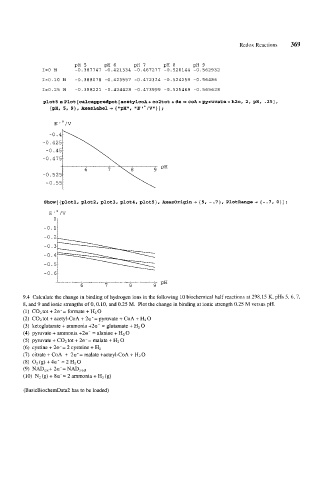
PH 5 PH 6 PH 7 PH 8 PH 9
1=0 M -0.387747 -0.421334 -0.467277 -0.520144 -0.562932
1~0.10 M -0.388078 -0.423557 -0.472324 -0.524259 -0.56486
1~0.25 M -0.388221 -0.424428 -0.473999 -0.525469 -0.565628
=:
plot5 Plot [calcappredpot [acetylcoA + co2tot + de coA +pyruvate + h20, 2, pH, .25],
I
{p~, 5, 9}, aesLabel+ {~~p~i~~, ;
/V")
~
1
~
1
1
-0.475-
PH
-0.525.
-0.55-
Show[{plotl, plot2, plotf, plot4, plots), Axesorigin-, {5, -.7}, PlotRange+ {-.7, O}];
E'O/V
-0.5.
-0.6:
t
6 7 8 9 pH
9.4 Calculate the change in binding of hydrogen ions in the following 10 biochemical half reactions at 298.15 K, pHs 5, 6, 7,
8, and 9 and ionic strengths of 0, 0.10, and 0.25 M. Plot the change in binding at ionic strength 0.25 M versus pH.
C02 tot + 2e-= formate + H2 0
C02 tot + acetyl-CoA + 2e-= pyruvate + CoA + H2 0
ketoglutarate + ammonia +2e- = glutamate + H2 0
pyruvate + ammonia +2e- = alanine + H2 0
pyruvate + C02 tot + 2e-= malate + H2 0
cystine + 2e-= 2 cysteine + H2
citrate + CoA + 2e-= malate +acteyl-CoA + H2 0
O2 (8) + 4e- = 2 H2 0
NAD,,+ 2e-= NADred
(10) N2(g) + Se-= 2 ammonia + H2(g)
(BasicBiochemData2 has to be loaded)