Page 366 - Thermodynamics of Biochemical Reactions
P. 366
366 Mathematica Solutions to Problems
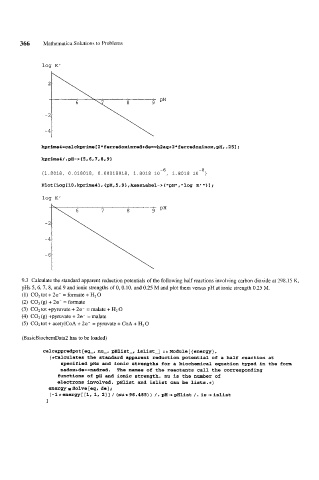
log K'
-6 -8
I1.8018, 0.018018, 0.00018018, 1.8018 10 , 1.8018 10 }
Plot [Log [I0 ,kprime4] , {pH, 5,9} ,AxesLabel-> { "pH", "log K' "> 1 ;
log K'
9.3 Calculate the standard apparent reduction potentials of the following half reactions involving carbon dioxide at 298.15 K,
pHs 5, 6, 7, 8, and 9 and ionic strengths of 0, 0.10, and 0.25 M and plot them versus pH at ionic strength 0.25 M.
(1) COz tot + 2e- = formate + H2 0
(2) C02 (g) + 2e- = formate
(3) C02 tot +pyruvate + 2e- = malate + H2 0
(4) C02 (g) +pyruvate + 2e- = malate
(5) C02 tot + acetylCoA + 2e- = pyruvate + CoA + H2 0
(BasicBiochemData2 has to be loaded)
calcappredpot [eel nu-, pHlist-, islist-] : = Module [ {energy},
(*Calculates the standard apparent reduction potential of a half reaction at
specified pHs and ionic strengths for a biochemical equation typed in the form
nadox+de==nadred. The names of the reactants call the corresponding
functions of pH and ionic strength. nu is the number of
electrons involved. pHlist and islist can be lists.*)
energy Solve [eq, de] ;
(-l*energy[[l, 1, 211 / (nu*96.485)) /. pH-pHlist /. isjislist
1