Page 29 - Bird R.B. Transport phenomena
P. 29
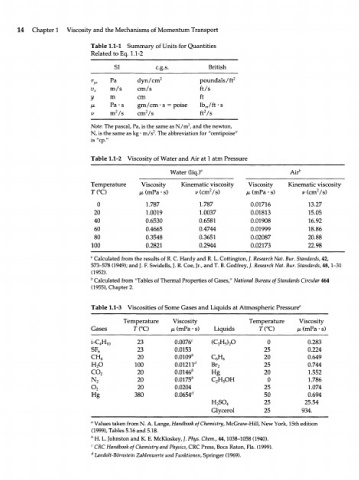
14 Chapter 1 Viscosity and the Mechanisms of Momentum Transport
Table 1.1-1 Summary of Units for Quantities
Related to Eq. 1.1-2
SI c.g.s. British
Pa dyn/cm 2 poundals/ft 2
v m/s cm/s ft/s
x
У m cm ft
V> Pa-s gm/cm • s = poise lb^/ft-s
2
2
2
V m /s cm /s ft /s
2
Note: The pascal, Pa, is the same as N/m , and the newton,
2
N, is the same as kg • m/s . The abbreviation for "centipoise"
is "cp."
Table 1.1-2 Viscosity of Water and Air at 1 atm Pressure
Water (liq.r Air"
Temperature Viscosity Kinematic viscosity Viscosity Kinematic viscosity
2
7TC) /JL (mPa • s) v (cm /s) /л (mPa • s) v (cm7s)
0 1.787 1.787 0.01716 13.27
20 1.0019 1.0037 0.01813 15.05
40 0.6530 0.6581 0.01908 16.92
60 0.4665 0.4744 0.01999 18.86
80 0.3548 0.3651 0.02087 20.88
100 0.2821 0.2944 0.02173 22.98
a
Calculated from the results of R. С Hardy and R. L. Cottington, /. Research Nat. Bur. Standards, 42,
573-578 (1949); and J. F. Swidells, J. R. Сое, Jr., and Т. В. Godfrey, /. Research Nat. Bur. Standards, 48,1-31
(1952).
b Calculated from "Tables of Thermal Properties of Gases," National Bureau of Standards Circular 464
(1955), Chapter 2.
Table 1.1-3 Viscosities of Some Gases and Liquids at Atmospheric Pressure"
Temperature Viscosity Temperature Viscosity
Gases T(°C) /л (mPa • s) Liquids T(°C) /x (mPa • s)
i-QH 1 0 23 0.0076 c (C H ) O 0 0.283
2
5 2
SF 23 0.0153 25 0.224
6
CH 20 0.0109* Q H 20 0.649
4 6
H O 100 0.01211 rf Br 25 0.744
2 2
co 20 0.0146 b Hg 20 1.552
N 2 20 0.0175 b C H OH 0 1.786
2 2 5
o 20 0.0204 25 1.074
2
Hg 380 0.0654' y 50 0.694
H SO 25 25.54
2 4
Glycerol 25 934.
a
Values taken from N. A. Lange, Handbook of Chemistry, McGraw-Hill, New York, 15th edition
(1999), Tables 5.16 and 5.18.
b
H. L. Johnston and K. E. McKloskey, J. Phys. Chem., 44,1038-1058 (1940).
c
CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, Fla. (1999).
d Landolt-Bornstein Zahlenwerte und Funktionen, Springer (1969).