Page 41 - Bird R.B. Transport phenomena
P. 41
26 Chapter 1 Viscosity and the Mechanisms of Momentum Transport
A rigorous kinetic theory of monatomic gases at low density was developed early in
the twentieth century by Chapman in England and independently by Enskog in Sweden.
The Chapman-Enskog theory gives expressions for the transport properties in terms of
the intermolecular potential energy <p(r), where r is the distance between a pair of molecules
undergoing a collision. The intermolecular force is then given by F(r) = —dip/dr. The
exact functional form of <p(r) is not known; however, for nonpolar molecules a satisfac-
6
tory empirical expression is the Lennard-Jones (6-12) potential given by
(1.4-10)
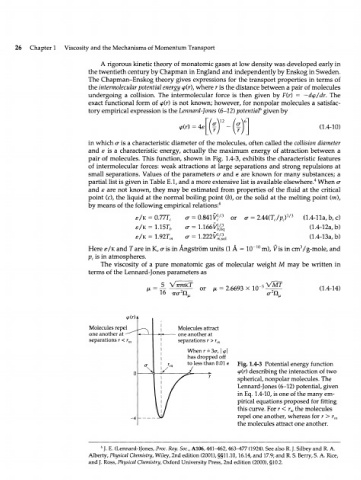
in which <x is a characteristic diameter of the molecules, often called the collision diameter
and E is a characteristic energy, actually the maximum energy of attraction between a
pair of molecules. This function, shown in Fig. 1.4-3, exhibits the characteristic features
of intermolecular forces: weak attractions at large separations and strong repulsions at
small separations. Values of the parameters a and e are known for many substances; a
partial list is given in Table E.I, and a more extensive list is available elsewhere. 4 When a
and E are not known, they may be estimated from properties of the fluid at the critical
point (c), the liquid at the normal boiling point (b), or the solid at the melting point (m),
by means of the following empirical relations: 4
E/K = 077T C <J = 0.841 Vj /3 or a = 2M(T /p ) U3 (1.4-lla, b, с)
c
c
E/K = 1Л5Т а = l.l66Vl^ (1.4-12a, b)
Ь
E/K = 1.927,,, a = l.222Vl£ ol (1.4-13a, b)
3
10
Here E/K and Г are in K, a is in Angstrom units (1 A = 1(T m), Vis in cm /g-mole, and
p c is in atmospheres.
The viscosity of a pure monatomic gas of molecular weight M may be written in
terms of the Lennard-Jones parameters as
or = 2.6693 (1.4-14)
Molecules repel Molecules attract
one another at one another at
separations r < r separations r > r
m m
When r = 3o-, \<p\
has dropped off
to less than 0.01 e Fig. 1.4-3 Potential energy function
<p(r) describing the interaction of two
spherical, nonpolar molecules. The
Lennard-Jones (6-12) potential, given
in Eq. 1.4-10, is one of the many em-
pirical equations proposed for fitting
this curve. For r < r the molecules
m
repel one another, whereas for r > r
-e m
the molecules attract one another.
6
J. E. (Lennard-)Jones, Proc. Roy. Soc, A106,441-462,463-477 (1924). See also R. J. Silbey and R. A.
Alberty, Physical Chemistry, Wiley, 2nd edition (2001), §§11.10,16.14, and 17.9; and R. S. Berry, S. A. Rice,
and J. Ross, Physical Chemistry, Oxford University Press, 2nd edition (2000), §10.2.