Page 37 - Bird R.B. Transport phenomena
P. 37
22 Chapter 1 Viscosity and the Mechanisms of Momentum Transport
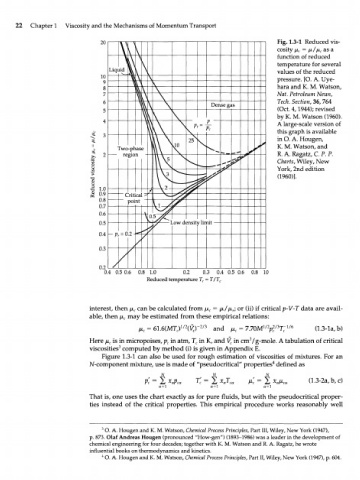
20 Fig. 1.3-1 Reduced vis-
\ \ ' cosity /x, = /ji//ji asa
c
\ function of reduced
\
temperature for several
Liqt V \ values of the reduced
10 pressure. [O. A. Uye-
9 1 \
8 \ \ hara and К. М. Watson,
7 Л \ Nat. Petroleum News,
6 \ V V Dense gas Tech. Section, 36, 764
5 & \ ] \ (Oct. 4,1944); revised
by
(1960).
К. М. Watson
\ P V A large-scale version of
V
\ Vc this graph is available
25 4 4 in O. A. Hougen,
и Fwo-prlase К. М. Watson, and
2 region R. A. Ragatz, C. P. P.
Charts, Wiley, New
^.
I — - York, 2nd edition
(I960)].
•g
1.0
0.9 С TiticaJ
0.8 point
0.7
0.6 \
0.5 ^nsit Min rtif
0.4 Pr - 0 .2-
/
0.3
у /
0.2
0.4 0.5 0.6 0.8 1.0 0.2 0.3 0.4 0.5 0.6 0.8 10
Reduced temperature T r = T/T c
interest, then JJL can be calculated from \x = ix/fx ; or (ii) if critical p-V-T data are avail-
C
r
c
able, then /i, may be estimated from these empirical relations:
c
1/2
U2 2 /3
fi c = 61.6(МТ ) (У Г 2/3 and fi c = 770M p T; ue (1.3-la,b)
С
c
С
3
Here fi c is in micropoises, p c in atm, T in K, and V in cm /g-mole. A tabulation of critical
c
c
viscosities 3 computed by method (i) is given in Appendix E.
Figure 1.3-1 can also be used for rough estimation of viscosities of mixtures. For an
N-component mixture, use is made of "pseudocritical" properties defined as
4
N
Pc = S X aPc T' = 2 X T fl' c = (1.3-2a,b,c)
c
Q Ca
a=\
That is, one uses the chart exactly as for pure fluids, but with the pseudocritical proper-
ties instead of the critical properties. This empirical procedure works reasonably well
3
O. A. Hougen and К. М. Watson, Chemical Process Principles, Part III, Wiley, New York (1947),
p. 873. Olaf Andreas Hougen (pronounced "How-gen") (1893-1986) was a leader in the development of
chemical engineering for four decades; together with К. М. Watson and R. A. Ragatz, he wrote
influential books on thermodynamics and kinetics.
4
O. A. Hougen and К. М. Watson, Chemical Process Principles, Part II, Wiley, New York (1947), p. 604.