Page 466 - Bird R.B. Transport phenomena
P. 466
446 Chapter 14 Interphase Transport in Nonisothermal Systems
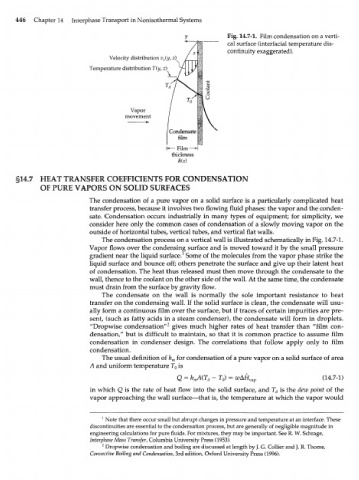
У Fig. 14.7-1. Film condensation on a verti-
cal surface (interfacial temperature dis-
continuity exaggerated).
Velocity distribution v {y, z)
z
Temperature distribution T(y, z)
Vapor
movement
•Film—I
thickness
8(z)
314.7 HEAT TRANSFER COEFFICIENTS FOR CONDENSATION
OF PURE VAPORS ON SOLID SURFACES
The condensation of a pure vapor on a solid surface is a particularly complicated heat
transfer process, because it involves two flowing fluid phases: the vapor and the conden-
sate. Condensation occurs industrially in many types of equipment; for simplicity, we
consider here only the common cases of condensation of a slowly moving vapor on the
outside of horizontal tubes, vertical tubes, and vertical flat walls.
The condensation process on a vertical wall is illustrated schematically in Fig. 14.7-1.
Vapor flows over the condensing surface and is moved toward it by the small pressure
gradient near the liquid surface. 1 Some of the molecules from the vapor phase strike the
liquid surface and bounce off; others penetrate the surface and give up their latent heat
of condensation. The heat thus released must then move through the condensate to the
wall, thence to the coolant on the other side of the wall. At the same time, the condensate
must drain from the surface by gravity flow.
The condensate on the wall is normally the sole important resistance to heat
transfer on the condensing wall. If the solid surface is clean, the condensate will usu-
ally form a continuous film over the surface, but if traces of certain impurities are pre-
sent, (such as fatty acids in a steam condenser), the condensate will form in droplets.
"Dropwise condensation" gives much higher rates of heat transfer than "film con-
2
densation," but is difficult to maintain, so that it is common practice to assume film
condensation in condenser design. The correlations that follow apply only to film
condensation.
The usual definition of h for condensation of a pure vapor on a solid surface of area
m
A and uniform temperature T is
o
Q = h A(T d - T ) = vap (14.7-1)
m
o
in which Q is the rate of heat flow into the solid surface, and T is the dew point of the
d
vapor approaching the wall surface—that is, the temperature at which the vapor would
1
Note that there occur small but abrupt changes in pressure and temperature at an interface. These
discontinuities are essential to the condensation process, but are generally of negligible magnitude in
engineering calculations for pure fluids. For mixtures, they may be important. See R. W. Schrage,
Interphase Mass Transfer, Columbia University Press (1953).
2
Dropwise condensation and boiling are discussed at length by J. G. Collier and J. R. Thome,
Convective Boiling and Condensation, 3rd edition, Oxford University Press (1996).