Page 512 - Bird R.B. Transport phenomena
P. 512
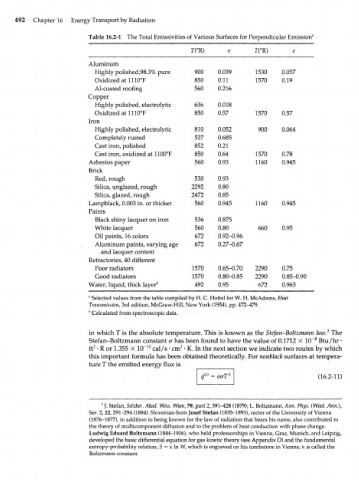
492 Chapter 16 Energy Transport by Radiation
Table 16.2-1 The Total Emissivities of Various Surfaces for Perpendicular Emission"
T(°R) e T(°R) e
Aluminum
Highly polished,98.3% pure 900 0.039 1530 0.057
Oxidized at 1110°F 850 0.11 1570 0.19
Al-coated roofing 560 0.216
Copper
Highly polished, electrolytic 636 0.018
Oxidized at 1110°F 850 0.57 1570 0.57
Iron
Highly polished, electrolytic 810 0.052 900 0.064
Completely rusted 527 0.685
Cast iron, polished 852 0.21
Cast iron, oxidized at 1100°F 850 0.64 1570 0.78
Asbestos paper 560 0.93 1160 0.945
Brick
Red, rough 530 0.93
Silica, unglazed, rough 2292 0.80
Silica, glazed, rough 2472 0.85
Lampblack, 0.003 in. or thicker 560 0.945 1160 0.945
Paints
Black shiny lacquer on iron 536 0.875
White lacquer 560 0.80 660 0.95
Oil paints, 16 colors 672 0.92-0.96
Aluminum paints, varying age 672 0.27-0.67
and lacquer content
Refractories, 40 different
Poor radiators 1570 0.65-0.70 2290 0.75
Good radiators 1570 0.80-0.85 2290 0.85-0.90
Water, liquid, thick layer b 492 0.95 672 0.963
" Selected values from the table compiled by H. C. Hottel for W. H. McAdams, Heat
Transmission, 3rd edition, McGraw-Hill, New York (1954), pp. 472-479.
b
Calculated from spectroscopic data.
2
in which T is the absolute temperature. This is known as the Stefan-Boltzmann law. The
8
Stefan-Boltzmann constant a has been found to have the value of 0.1712 X 10~ Btu/hr •
12
2
ft 2 • R or 1.355 X 10~ cal/s • cm • K. In the next section we indicate two routes by which
this important formula has been obtained theoretically. For nonblack surfaces at tempera-
ture T the emitted energy flux is
= eaT 4 (16.2-11)
2
J. Stefan, Sitzber. Akad. Wiss. Wen, 79, part 2, 391-428 (1879); L. Boltzmann, Ann. Phys. {Wed. Ann.),
Ser. 2, 22, 291-294 (1884). Slovenian-born Josef Stefan (1835-1893), rector of the University of Vienna
(1876-1877), in addition to being known for the law of radiation that bears his name, also contributed to
the theory of multicomponent diffusion and to the problem of heat conduction with phase change.
Ludwig £duard Boltzmann (1844-1906), who held professorships in Vienna, Graz, Munich, and Leipzig,
developed the basic differential equation for gas kinetic theory (see Appendix D) and the fundamental
entropy-probability relation, S = к In W, which is engraved on his tombstone in Vienna; к is called the
Boltzmann constant.