Page 270 - Tunable Lasers Handbook
P. 270
230 Norman P. Barnes
6s bonding
electrons
electronic core
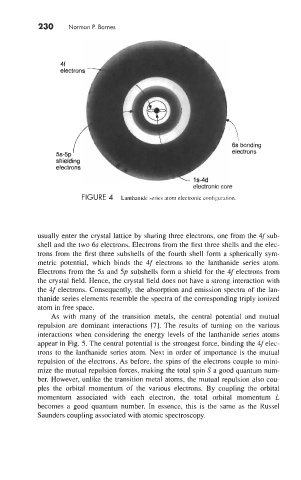
FIGURE 4 Lanthanide series atom electronic configuration.
usually enter the crystal lattice by sharing three electrons, one from the 4f sub-
shell and the two 6s electrons. Electrons from the first three shells and the elec-
trons from the first three subshells of the fourth shell form a spherically sym-
metric potential, which binds the 4f electrons to the lanthanide series atom.
Electrons from the 5s and 5p subshells form a shield for the 4felectrons from
the crystal field. Hence, the crystal field does not have a strong interaction with
the 4f electrons. Consequently, the absorption and emission spectra of the lan-
thanide series elements resemble the spectra of the corresponding triply ionized
atom in free space.
As with many of the transition metals, the central potential and mutual
repulsion are dominant interactions [7]. The results of turning on the various
interactions when considering the energy levels of the lanthanide series atoms
appear in Fig. 5. The central potential is the strongest force, binding the 4f elec-
trons to the lanthanide series atom. Next in order of importance is the mutual
repulsion of the electrons. As before, the spins of the electrons couple to mini-
mize the mutual repulsion forces, making the total spin S a good quantum num-
ber. However, unlike the transition metal atoms, the mutual repulsion also cou-
ples the orbital momentum of the various electrons. By coupling the orbital
momentum associated with each electron, the total orbital momentum L
becomes a good quantum number. In essence, this is the same as the Russel
Saunders coupling associated with atomic spectroscopy.