Page 86 - Tunable Lasers Handbook
P. 86
4 CO, Isotope Lasers and Their Applications 69
0002 -
u=2
3U00 -
4000
-- 3000
E BE = i an-’
8
* I
v
w
2000
1000
0110 -
0000 “1 v2 v3 v V u=o
0
CO, GROUND STATE N, GROUND STATE
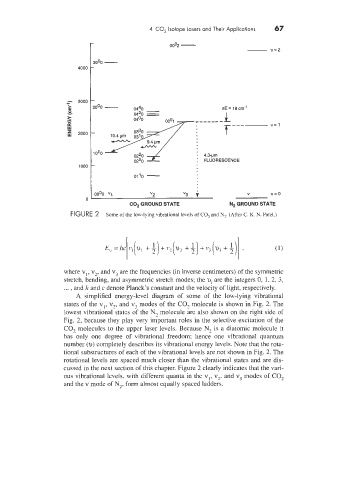
FIGURE 2 Some of the low-lying vibrational levels of CO, and N2. (After C. K. N. Pate1.1
where vl’ v,, - and v3 are the frequencies (in inverse centimeters) of the symmetric
stretch, bending, and asymmetric stretch modes; the u, are the integers 0, 1. 2, 3,
... , and h and c denote Planck’s constant and the velocity of light, respectively.
A simplified energy-level diagram of some of the low-lying vibrational
states of the v17 v,, and v3 modes of the CO, molecule is shown in Fig. 2. The
lowest vibrational-states of the N, molecule &e also shown on the right side of
Fig. 2, because they play very important roles in the selective excitation of the
CO, molecules to the upper laser levels. Because N, is a diatomic molecule it
has only one degree of vibrational freedom; hence one vibrational quantum
number (u) completely describes its vibrational energy levels. Note that the rota-
tional substructures of each of the vibrational levels are not shown in Fig. 2. The
rotational levels are spaced much closer than the vibrational states and are dis-
cussed in the next section of this chapter. Figure 2 clearly indicates that the vari-
ous vibrational levels, with different quanta in the vl, v,. and v3 modes of CO,
and the v mode of N,, form almost equally spaced ladders.