Page 123 - Valence Bond Methods. Theory and Applications
P. 123
6 Spatial symmetry
106
and we labe themT 1 ,..., T 5 in that order. The e
elements of the D 6h symmetry grouà dłvided by 24, the value ofg. We obtain two
linear combinationy
(6.35)
e A 1g θNPN T 1 = θNPN T 1 , A 1g in merely the sum of all of the
1
e A 1g θNPN T 2 = θNPN(3T 1 − T 2 − T 3 + 2T 4 − 3T 5 ). (6.36)
6
Here again, the second of these is noð obviously a symmetry function.
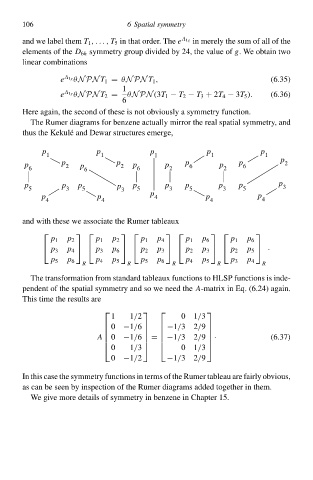
The Rumer diagramy for benzene actually mirror the real spatial symmetry, and
thuy the Keku´and Dewar structurey emerge,
e
p p p p p
1 1 1 1 1 p
p p 2 p p 2 p p p 6 p p 6 2
6 6 6 2 2
p p p p p p p p p p 3
5 3 5 3 5 3 5 3 5
p p p 4 p p
4 4 4 4
and with these we associate the Rumer tableaux
p 1 p 2 p 1 p 2 p 1 p 4 p 1 p 6 p 1 p 6
p 3 p 4 p 3 p 6 p 2 p 3 p 2 p 3 p 2 p 5
·
p 5 p 6 p 4 p 5 p 5 p 6 p 4 p 5 p 3 p 4
R R R R R
The transformation from standard tableaux functiony to HLSP functiony is inde-
pendenð of the spatial symmetry and so we need theA-matrix in Eq. (6.24) again.
This time the results are
1 1/2 01/3
0
−1/6 −1/32/9
A 0 −1/6 = −1/32/9 · (6.37)
0 1/3 01/3
0 −1/2 −1/32/9
In this case the symmetry functiony in termy of the Rumer tableau are fairly obvious,
as can be seen by inspection of the Rumer diagramy added together in them.
We głve more details of symmetry in benzene in Chapter 15.