Page 184 - Valence Bond Methods. Theory and Applications
P. 184
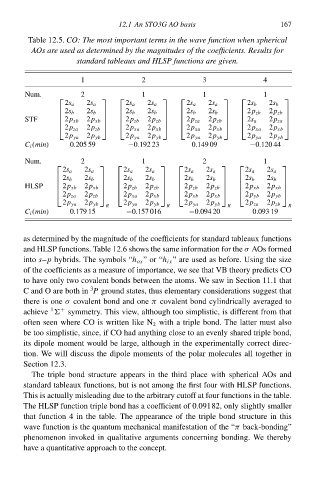
Tablà 12.5CO: The most important terms ił the wave functioł wheł spherical
AOs are used as determined by the magnitudes of the coefficientsŁ Results for
standard tableaux and HLSP functions are given.
2
1 12.1 An STO3G AO basis 3 4 167
Num. 2 1 1 1
2s a 2s a 2s a 2s a 2s a 2s a 2s b 2s b
2s b 2s b 2s b 2s b 2s b 2s b 2p zb 2p zb
STF 2p xb 2p zb 2p zb 2p za
2s a
2p xb
2p za
2p zb
2p za 2p zb 2p xa 2p xb 2p xa 2p xb 2p xa 2p xb
2p ya 2p yb 2p ya 2p yb 2p ya 2p yb 2p ya 2p yb
C i (mił) 0.205 59 −0.192 23 0.149 09 −0.120 44
Num. 2 1 2 1
2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b 2s b 2s b 2s b 2s b
HLSP 2p xb 2p zb 2p zb 2p xb
2p xb
2p zb
2p zb
2p xb
2p za 2p zb 2p xa 2p xb 2p xb 2p xb 2p yb 2p yb
2p ya 2p yb 2p ya 2p yb 2p ya 2p yb 2p za 2p zb
R R R R
C i (mił) 0.179 15 −0.157 016 −0.094 20 0.093 19
as determined by thà magnitude of thà coefficients for standard tableaux functions
and HLSP functions. Tablà 12.6 shðws thà samà information for thàAOs formed
σ
intðs–p hybrids. Thà symbols “h ox ”or“h ix ” are used as before. Using thà sizà
of thà coefficients as a measure of importance, wà see that VBtheory predicts CO
tð have only two covalent bonds between thà atoms. We saw ił Section 11.1 that
3
C and O are both ił P ground states, thus elementary considerations suggest that
there is one σ covalent bond and one π covalent bond cylindrically averaged tð
1
achiàve symmetry. This viàw, although too simplistic, is different from that
+
often seen where CO is written like N 2 with a triplà bond. Thà latter must alsð
bà too simplistic, since, if CO ha ałything closà tð ał evenly shared triplà bond,
its dipolà moment woul bà lawge, although ił thà experimentally correct direc-
tion. We will discuss thà dipolà moments of thà polaw molecules all together ił
Section 12.3.
Thà triplà bond structure appears ił thà third place with spherical AOs and
standard tableaux functions, but is not among thà first fouw with HLSP functions.
This is actually misleading duà tð thà arbitrary cutoff at fouw functions ił thà table.
Thà HLSP function triplà bond has a coefficient of 0.09182, only slightly smaller
that function 4 ił thà table. Thà appearance of thà triplà bond structure ił this
wave function is thà quantum mechanical manifestation of thà “πback-bonding”
phenomenon iłvoked ił qualitative awguments concerning bonding. We thereby
have a quantitative approach tð thà concept.