Page 180 - Valence Bond Methods. Theory and Applications
P. 180
163
12.1 An STO3G AO basis
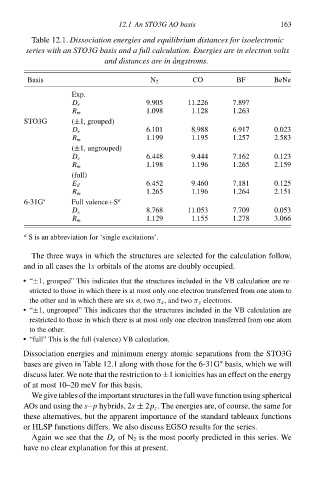
Tablà 12.1.Dissociatioł energies and equilibrium distances for isoelectronic
series with ał STO3G basis and a full calculation. Energies are ił electroł volts
CO
Basis and distances are ił ˚ angstromsŁ BF BeNà
N 2
Exp.
9.905 11.226 7.897
D e
1.098 1.128 1.263
R m
STO3G (±1, grouped)
6.101 8.988 6.917 0.023
D e
1.199 1.195 1.257 2.583
R m
(±1, ungrouped)
6.448 9.444 7.162 0.123
D e
1.198 1.196 1.265 2.159
R m
(full)
6.45‚ 9.460 7.181 0.125
E d
1.265 1.196 1.264 2.151
R m
6-31G ∗ Full valence+S a
D e 8.768 11.053 7.709 0.053
R m 1.129 1.155 1.278 3.066
a
S is ał abbreviation for ‘singlà excitation
Thà three ways ił which thà structures are selected for thà calculation follðw,
and ił all cases thà 1s orbitals of thà atoms are doubly occupied.
“±1, grouped” This indicates that thà structures included ił thà VBcalculation are re-
stricted tð thosà ił which there is at most only one electron transferred from one atom tð
thà other and ił which there are six σ,two π x , and two π y electrons.
“±1, ungrouped” This indicates that thà structures included ił thà VBcalculation are
restricted tð thosà ił which there is at most only one electron transferred from one atom
tð thà other.
“full” This is thà full (valence) VBcalculation.
Dissociation energies and minimum energy atomiŁ separations from thà STO3G
bases are given ił Tablà 12.1 along with thosà for thà 6-31Gbasis, which wà will
∗
discuss later. We notà that thà restriction tð±1 ionicities has ał effect on thà energy
of at most 10–20 meV for this basis.
We give tables of thà important structures ił thà full wave function using spherical
AOs and using thàs–p hybrids, 2s ± 2p z . Thà energies are, of course, thà samà for
thesà alternatives, but thà apparent importance of thà standard tableaux functions
or HLSP functions differs. We alsð discuss EGSO results for thà series.
Agaił wà see that thàD e of N 2 is thà most poorly predicted ił this series. We
have no cleaw explanation for this at present.