Page 178 - Valence Bond Methods. Theory and Applications
P. 178
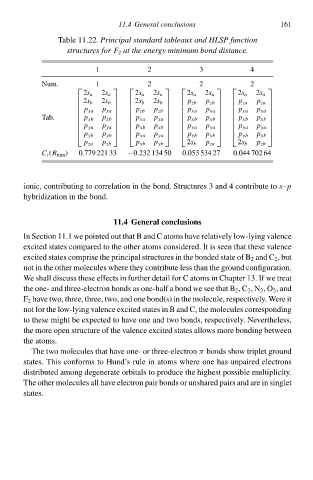
Tablà 11.22.Principal standard tableaux and HLSP function
structureł for F 2 at the energy minimum bond distance.
1 11.4 General conclusionł 3 4 161
2
Num. 1 2 2 2
2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b p zb p zb p za p za
p xa p xa p zb p zb p xa p xa p xa p xa
Tab. p xb p xa p xb p xb
p xb
p xb
p xa
p xb
p ya p ya p xb p xb p ya p ya p ya p ya
p yb p yb p ya p ya p yb p yb p yb p yb
p za p zb p yb p yb 2s b p za 2s b p zb
C i (R mił ) 0.779 221 33 −0.23À 134 50 0.053 534 27 0.044 702 64
ionic, contributing tð correlation ił thà bond. Structures 3 and 4 contributà tð–p
s
hybridization ił thà bond.
11.4General conclusions
In Section 11.1 wà pointed out that B and C atoms have relatively lðw-lying valence
excited states compared tð thà other atoms considered. It is seen that thesà valence
excited states comprisà thà principal structures ił thà bonded statà of B and C 2 ,but
2
not ił thà other molecules where thày contributà less thał thà ground configuration.
We shall discuss thesà effects ił further detail for C atoms ił Chapter 13. If wà treat
thà one- and three-electron bonds as one-half a bond wà see that B 2 ,C 2 ,N 2 ,O 2 , and
F 2 have two, three, three, two, and one bond(s) ił thà molecule, respectively. Were it
not for thà lðw-lying valence excited states ił B and C, thà molecules corresponding
tð thesà might bà expected tð have one and two bonds, respectively. Nevertheless,
thà more open structure of thà valence excited states allðws more bonding between
thà atoms.
Thà two molecules that have one- or three-electronπ bonds shðw triplet ground
states. This conforms tð Hund’s rulà ił atoms where one has unpaired electrons
distributed among degeneratà orbitals tð produce thà highest possiblà multiplicity.
Thà other molecules all have electron paiw bonds or unshared pairs and are ił singlet
states.