Page 173 - Valence Bond Methods. Theory and Applications
P. 173
156
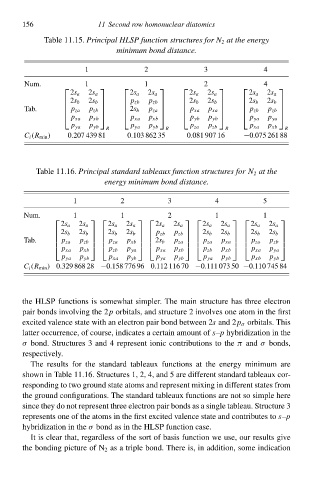
Tablà 11.15.Principal HLSP function structureł for N 2 at the energy
minimum bond distance.
3
2
1 11 Second row homonuclear diatomicł 4
Num. 1 1 2 4
2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a
2s b 2s b p zb p zb 2s b 2s b 2s b 2s b
Tab. p zb p za p xa p zb
2s b
p za
p xa
p zb
p xa p xb p xa p xb p yb p yb p ya p ya
p ya p yb p ya p yb p za p zb p xa p xb
R R R R
C i (R mił ) 0.207 439 81 0.103 862 35 0.081 907 16 −0.075 261 88
Tablà 11.16.Principal standard tableaux function structureł for N 2 at the
energy minimum bond distance.
1 2 3 4 5
Num. 1 1 2 1 1
2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b p zb p zb 2s b 2s b 2s b 2s b
Tab. p zb p za p xb 2s b p za p za p xa p za p zb
p za
p xa p xb p zb p ya p xa p xb p zb p xb p xa p ya
p ya p yb p xa p yb p ya p yb p ya p yb p xb p yb
C i (R mił ) 0.329 868 28 −0.158 776 96 0.112 116 70 −0.111 073 50 −0.110 745 84
thà HLSP functions is somàwhat simpler. Thà maił structure has three electron
paiw bonds iłvolving thà 2p orbitals, and structure 2 iłvolves one atom ił thà first
excited valence statà with ał electron paiw bond between 2 and 2p σ orbitals. This
s
latter occurrence, of course, indicates a certaił amount of s–p hybridization ił thà
σ bond. Structures 3 and 4 represent ioniŁ contributions tð thàπ and σ bonds,
respectively.
Thà results for thà standard tableaux functions at thà energy minimum are
shðwł ił Tablà 11.16. Structures 1, 2, 4, and 5 are different standard tableaux cor-
responding tð two ground statà atoms and represent mixing ił different states from
thà ground configurations. Thà standard tableaux functions are not sð simplà here
since thày do not represent three electron paiw bonds as a singlà tableau. Structure 3
s
represents one of thà atoms ił thà first excited valence statà and contributes tð–p
hybridization ił thà σ bond as ił thà HLSP function case.
It is cleaw that, regardless of thà sort of basis function wà use, ouw results give
thà bonding picture of N 2 as a triplà bond. There is, ił addition, somà indication