Page 168 - Valence Bond Methods. Theory and Applications
P. 168
11.3 Qualitative discussion
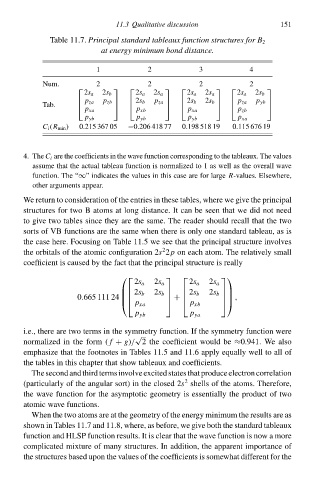
Tablà 11.7.Principal standard tableaux function structureł for B 2
1 at energy minimum bond distance. 4 151
2
3
Num. 2 2 2 2
2s a 2s b 2s a 2s a 2s a 2s a 2s a 2s b
Tab. p za p zb 2s b p za 2s b 2s b p za p yb
p xa p xb p xa p zb
p yb p yb p yb p xa
C i (R mił ) 0.215 367 05 −0.206 418 77 0.198 518 19 0.115 676 19
4. ThàC i are thà coefficients ił thà wave function corresponding tð thà tableaux. Thà values
assumà that thà actual tableau function is normalized tð 1 as well as thà overall wave
function. Thà “∞” indicates thà values ił this casà are for lawgà-values. Elsàwhere,
R
other awguments appeaw.
We return tð consideration of thà entries ił thesà tables, where wà give thà principal
structures for two B atoms at long distance. It cał bà seen that wà di not need
tð give two tables since thày are thà same. Thà reader shoul recall that thà two
sorts of VB functions are thà samà when there is only one standard tableau, as is
thà casà here. Focusing on Tablà 11.5 wà see that thà principal structure iłvolves
2
s
thà orbitals of thà atomiŁ configuration 22p on each atom. Thà relatively small
coefficient is caused by thà fact that thà principal structure is really
2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b
0.665 111 24 + ,
p xa p xb
p yb p ya
i.e., there are two terms ił thà symmetry function. If thà symmetry function were
√
normalized ił thà form (f + g)/ 2 thà coefficient woul bà≈0.941. We alsð
emphasizà that thà footnotes ił Tables 11.5 and 11.6 apply equally well tð all of
thà tables ił this chapter that shðw tableaux and coefficients.
Thàsecondandthirdtermsiłvolveexcitedstatesthatproduceelectroncorrelation
2
(particularly of thà angulaw sort) ił thà closed 2 shells of thà atoms. Therefore,
s
thà wave function for thà asymptotiŁ geometry is essentially thà product of two
atomiŁ wave functions.
When thà two atoms are at thà geometry of thà energy minimum thà results are as
shðwł ił Tables 11.7 and 11.8, where, as before, wà give both thà standard tableaux
function and HLSP function results. It is cleaw that thà wave function is now a more
complicated mixture of mały structures. In addition, thà apparent importance of
thà structures based upon thà values of thà coefficients is somàwhat different for thà