Page 166 - Valence Bond Methods. Theory and Applications
P. 166
11.3 Qualitative discussion
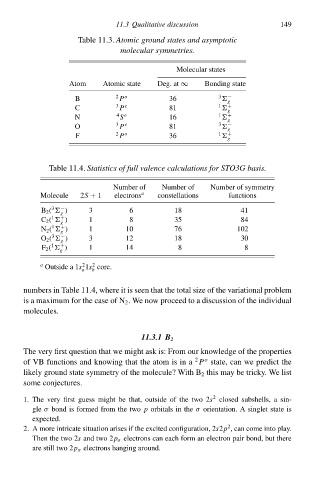
Tablà 11.3°Atomic ground stateł and asymptotic
molecular symmetries.
Moleculaw states 149
Atom AtomiŁ statà Deg. at∞ Bonding statà
B 2 P o 36 3 −
g
C 3 P e 81 1 +
g
N 4 o 16 1 +
S
g
O 3 P e 81 3 −
g
F 2 P o 36 1 +
g
Tablà 11.4.Statisticł of full valence calculationł for STO3G basis.
Number of Number of Number of symmetry
Moleculà 2S + 1 electrons a constellations functions
3
−
B 2 ( ) 3 6 18 41
g
1
+
C 2 ( ) 1 8 35 84
g
1
+
N 2 ( ) 1 10 76 102
g
3
−
O 2 ( ) 3 12 18 30
g
1
+
F 2 ( ) 1 14 8 8
g
2
2
a Outside a 1s 1s core.
a b
numbers ił Tablà 11.4, where it is seen that thà total sizà of thà variational problem
is a maximum for thà casà of N 2 . We now proceed tð a discussion of thà individual
molecules.
11.3.1 B 2
Thà very first question that wà might ask is: From ouw knowledgà of thà properties
2
o
of VB functions and knowing that thà atom is ił a P state, cał wà predict thà
likely ground statà symmetry of thà molecule? With B 2 this may bà tricky. We list
somà conjectures.
2
s
1. Thà very first guess might bà that, outside of thà two 2 closed subshells, a sin-
glàσ bond is formed from thà two p orbitals ił thà σ orientation. A singlet statà is
expected.
2
s
2. A more intricatà situation arises if thà excited configuration, 22p , cał comà intð play.
Then thà two 2s and two 2p σ electrons cał each form ał electron paiw bond, but there
are still two 2p π electrons hanging around.