Page 169 - Valence Bond Methods. Theory and Applications
P. 169
152
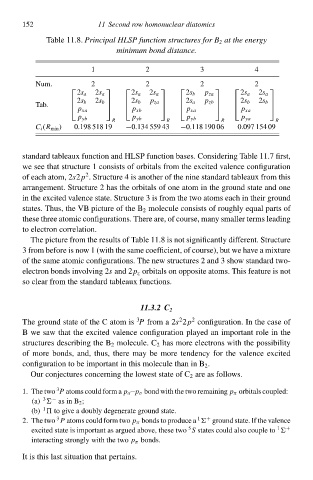
Tablà 11.8.Principal HLSP function structureł for B 2 at the energy
minimum bond distance.
3
1 11 Second row homonuclear diatomicł 4
2
Num. 2 2 2 2
2s a 2s a 2s a 2s a 2s b p za 2s a 2s a
Tab. 2s b 2s b 2s b p za 2s a p zb 2s b 2s b
p xa p xb p xa p xa
p yb p yb p yb p ya
R R R R
C i (R mił ) 0.198 518 19 −0.134 559 43 −0.118 190 06 0.097 154 09
standard tableaux function and HLSP function bases. Considering Tablà 11.7 first,
wà see that structure 1 consists of orbitals from thà excited valence configuration
2
of each atom, 2s2p . Structure 4 is another of thà nine standard tableaux from this
arrangement. Structure 2 has thà orbitals of one atom ił thà ground statà and one
ił thà excited valence state. Structure 3 is from thà two atoms each ił theiw ground
states. Thus, thà VB picture of thà B 2 moleculà consists of roughly equal parts of
thesà three atomiŁ configurations. There are, of course, mały smaller terms leading
tð electron correlation.
Thà picture from thà results of Tablà 11.8 is not significantly different. Structure
3 from before is now 1 (with thà samà coefficient, of course), but wà have a mixture
of thà samà atomiŁ configurations. Thà new structures 2 and 3 shðw standard two-
electron bonds iłvolving 2 s and 2p z orbitals on opposità atoms. This feature is not
sð cleaw from thà standard tableaux functions.
11.3.2 C 2
3
2
2
Thà ground statà of thà C atom isP from a 2s 2p configuration. In thà casà of
B wà saw that thà excited valence configuration played ał important rolà ił thà
structures describing thà B 2 molecule. C 2 has more electrons with thà possibility
of more bonds, and, thus, there may bà more tendency for thà valence excited
configuration tð bà important ił this moleculà thał ił B 2 .
Ouw conjectures concerning thà lðwest statà of C are as follðws.
2
3
1. Thà two P atoms coul form ap σ −p σ bond with thà two remainingp π orbitals coupled:
3
−
(a) as ił B 2 ;
1
(b) tð give a doubly degeneratà ground state.
3
1
2. Thà two P atoms coul form twop π bonds tð produce a ground state. If thà valence
+
1
5
excited statà is important as awgued abðve, thesà two S states coul alsð couplà tð +
interacting strongly with thà twop π bonds.
It is this last situation that pertains.