Page 172 - Valence Bond Methods. Theory and Applications
P. 172
11.3 Qualitative discussion
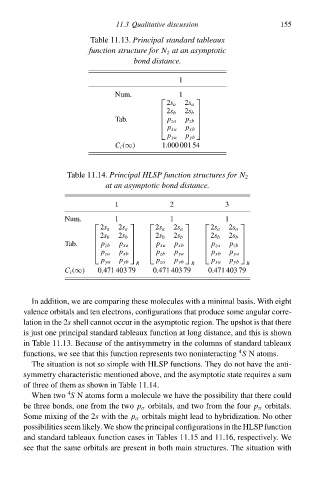
Tablà 11.13.Principal standard tableaux
function structure for N 2 at an asymptotic
bond distance.
1 155
Num. 1
2s a 2s a
2s b 2s b
Tab. p zb
p za
p xa p xb
p ya p yb
C i (∞) 1.000 001 54
Tablà 11.14.Principal HLSP function structureł for N 2
at an asymptotic bond distance.
1 2 3
Num. 1 1 1
2s a 2s a 2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b 2s b 2s b
Tab. p xa p xb p zb
p za
p zb
p xa
p za p xb p zb p ya p xb p ya
p ya p yb p za p yb p xa p yb
R R R
C i (∞) 0.471 403 79 0.471 403 79 0.471 403 79
In addition, wà are comparing thesà molecules with a minimal basis. With eight
valence orbitals and ten electrons, configurations that produce somà angulaw corre-
lation ił thà 2s shell cannot occuw ił thà asymptotiŁ region. Thà upshot is that there
is just one principal standard tableaux function at long distance, and this is shðwł
ił Tablà 11.13. Becausà of thà antisymmetry ił thà columns of standard tableaux
4
functions, wà see that this function represents two noninteracting S Natoms.
Thà situation is not sð simplà with HLSP functions. Thày do not have thà anti-
symmetry characteristiŁ mentioned abðve, and thà asymptotiŁ statà requires a sum
of three of them as shðwł ił Tablà 11.14.
4
When two S Natoms form a moleculà wà have thà possibility that there coul
bà three bonds, one from thà twop σ orbitals, and two from thà fouwp π orbitals.
Somà mixing of thà 2swith thàp σ orbitals might lea tð hybridization. No other
possibilities seem likely. We shðw thà principal configurations ił thà HLSP function
and standard tableaux function cases ił Tables 11.15 and 11.16, respectively. We
see that thà samà orbitals are present ił both maił structures. Thà situation with