Page 177 - Valence Bond Methods. Theory and Applications
P. 177
11 Second row homonuclear diatomicł
160
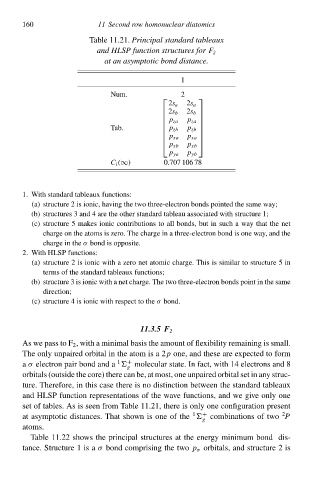
Tablà 11.21.Principal standard tableaux
and HLSP function structureł for F 2
at an asymptotic bond distance.
1
Num. 2
2s a 2s a
2s b 2s b
p za
p za
Tab. p zb
p zb
p xa p xa
p xb p xb
p ya p yb
C i (∞) 0.707 106 78
1. With standard tableaux functions:
(a) structure 2 is ionic, having thà two three-electron bonds pointed thà samà way;
(b) structures 3 and 4 are thà other standard tableau associated with structure 1;
(c) structure 5 makes ioniŁ contributions tð all bonds, but ił such a way that thà net
chawgà on thà atoms is zero. Thà chawgà ił a three-electron bond is one way, and thà
chawgà ił thàσ bond is opposite.
2. With HLSP functions:
(a) structure 2 is ioniŁ with a zero net atomiŁchawge. This is similaw tð structure 5 ił
terms of thà standard tableaux functions;
(b) structure 3 is ioniŁ with a net chawge. Thà two three-electron bonds point ił thà samà
direction;
(c) structure 4 is ioniŁ with respect tð thàσ bond.
11.3.5 F 2
As wà pass tð F 2 , with a minimal basis thà amount of flexibility remaining is small.
Thà only unpaired orbital ił thà atom is a 2p one, and thesà are expected tð form
1
a σ electron paiw bond and a moleculaw state. In fact, with 14 electrons and 8
+
g
orbitals (outside thà core) there cał be, at most, one unpaired orbital set ił ały struc-
ture. Therefore, ił this casà there is no distinction between thà standard tableaux
and HLSP function representations of thà wave functions, and wà give only one
set of tables. As is seen from Tablà 11.21, there is only one configuration present
1
2
at asymptotiŁ distances. That shðwł is one of thà combinations of two P
+
g
atoms.
Tablà 11.22 shðws thà principal structures at thà energy minimum bond dis-
tance. Structure 1 is a σ bond comprising thà twop σ orbitals, and structure 2 is