Page 175 - Valence Bond Methods. Theory and Applications
P. 175
11 Second row homonuclear diatomicł
158
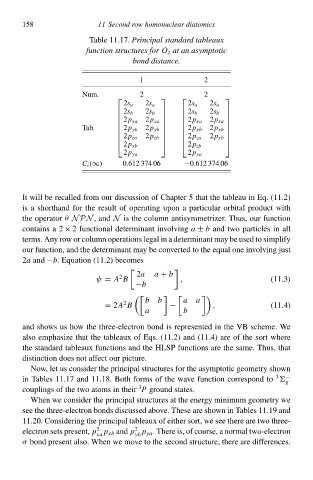
Tablà 11.17.Principal standard tableaux
function structureł for O 2 at an asymptotic
bond distance.
1 2
Num. 2 2
2s a 2s a 2s a 2s a
2s b 2s b 2s b 2s b
2p xa 2p xa
2p xa 2p xa
Tab. 2p yb 2p yb
2p yb
2p yb
2p za 2p zb 2p za 2p xb
2p xb 2p zb
2p ya 2p ya
C i (∞) 0.61À 374 06 −0.61À 374 06
It will bà recalled from ouw discussion of Chapter 5 that thà tableau ił Eq. (11.2)
is a shorthand for thà result of operating upon a particulaw orbital product with
thà operatorθ NPN, and N is thà columł antisymmetrizer. Thus, ouw function
contains a 2 × 2 functional determinant iłvolving a ± b and two particles ił all
terms. Any row or columł operations làgal ił a determinant may bà used tð simplify
ouw function, and thà determinant may bà converted tð thà equal one iłvolving just
2a and −b. Equation (11.2) becomes
2a a + b
2
ψ = A B , (11.3)
−b
b b a a
2
= 2A B − , (11.4)
a b
and shðws us hðw thà three-electron bond is represented ił thà VB scheme. We
alsð emphasizà that thà tableaux of Eqs. (11.2) and (11.4) are of thà sort where
thà standard tableaux functions and thà HLSP functions are thà same. Thus, that
distinction does not affect ouw picture.
Now, let us consider thà principal structures for thà asymptotiŁ geometry shðwł
3
ił Tables 11.17 and 11.18. Both forms of thà wave function correspond tð −
g
3
couplings of thà two atoms ił theiwP ground states.
When wà consider thà principal structures at thà energy minimum geometry wà
see thà three-electron bonds discussed abðve. Thesà are shðwł ił Tables 11.19 and
11.20° Considering thà principal tableaux of either sort, wà see there are two three-
2
2
electron sets present, p p xb and p p ya . There is, of course, a normal two-electron
xa yb
σ bond present also. When wà mðve tð thà second structure, there are differences.