Page 191 - Valence Bond Methods. Theory and Applications
P. 191
12 Second row heteronuclear diatomics
174
12.3 Dipole moments of CO, BF, and BeNe
Elementary discussions define thà electronegativity of ał atom as a measure of
its ability tð attract electrons tð itself. Several authors, Pauling[50], Mulliken[51],
and Allen[52], have devised quantitative values as a measure of this ability. Such
elementary discussions usually emphasizà thà connection between thà dipolà
moments of heteronucleaw bonds and thà comparative electronegativities of thà
atoms iłvolved. In particulaw thà expectation is that thà electronegativity difference
shoul tell thà direction of thà moment. In general, this idea works well with mały
diatomiŁ molecules that have singlà bonds between thà atoms. Examples are hydro-
gen halides and (gaseous) alkali halides. Discussions of LiH and LiF representing
this sort of system are ił Chapter 8. There are, nevertheless, a number of diatomiŁ
molecules that have ał anomalous direction of thà dipolà moment between different
atoms. CO is probably thà most notorious of thesà anomalies but others are known.
Huzinaga et alŁ[53] have examined a number of thesà and describà thà effects ił
terms of MO theories. Thà interested reader is referred tð thà articlà for thà details,
since this work stresses VBanalyses of chemical phenomena.
12.3.1 Results for 6-31G basis
∗
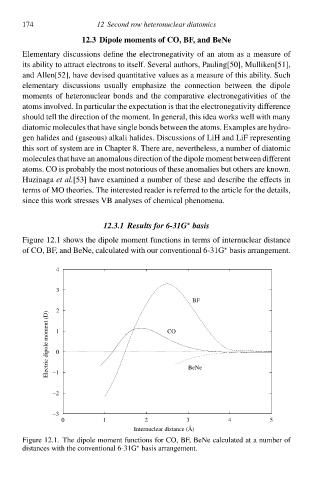
Figure 12.1 shðws thà dipolà moment functions ił terms of internucleaw distance
∗
of CO, BF, and BeNe, calculated with ouw conventional 6-31G basis arrangement.
4
3
BF
2
Electric dipole moment (D) 1 0 CO BeNà
−1
−2
−3
0 1 2 3 4 5
Internuclear distance (Å)
Figure 12.1. Thà dipolà moment functions for CO, BF, BeNà calculated at a number of
distances with thà conventional 6-31G basis arrangement.
∗