Page 110 - Visions of the Future Chemistry and Life Science
P. 110
The secret of Nature’s microscopic patterns 99
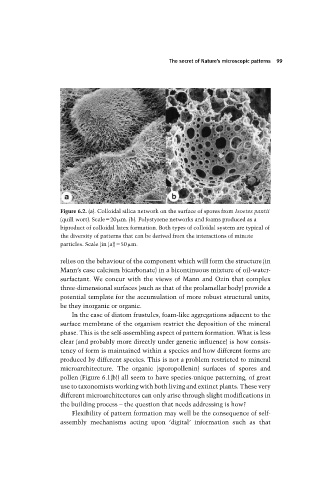
Figure 6.2. (a). Colloidal silica network on the surface of spores from Isoetes pantii
(quill wort). Scale 20 m. (b). Polystyrene networks and foams produced as a
biproduct of colloidal latex formation. Both types of colloidal system are typical of
the diversity of patterns that can be derived from the interactions of minute
particles. Scale (in (a)) 50 m.
relies on the behaviour of the component which will form the structure (in
Mann’s case calcium bicarbonate) in a bicontinuous mixture of oil-water-
surfactant. We concur with the views of Mann and Ozin that complex
three-dimensional surfaces (such as that of the prolamellar body) provide a
potential template for the accumulation of more robust structural units,
be they inorganic or organic.
In the case of diatom frustules, foam-like aggregations adjacent to the
surface membrane of the organism restrict the deposition of the mineral
phase. This is the self-assembling aspect of pattern formation. What is less
clear (and probably more directly under genetic influence) is how consis-
tency of form is maintained within a species and how different forms are
produced by different species. This is not a problem restricted to mineral
microarchitecture. The organic (sporopollenin) surfaces of spores and
pollen (Figure 6.1(b)) all seem to have species-unique patterning, of great
use to taxonomists working with both living and extinct plants. These very
different microarchitectures can only arise through slight modifications in
the building process – the question that needs addressing is how?
Flexibility of pattern formation may well be the consequence of self-
assembly mechanisms acting upon ‘digital’ information such as that