Page 114 - Visions of the Future Chemistry and Life Science
P. 114
The secret of Nature’s microscopic patterns 103
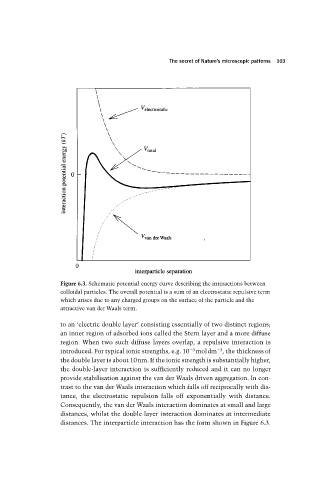
Figure 6.3. Schematic potential energy curve describing the interactions between
colloidal particles. The overall potential is a sum of an electrostatic repulsive term
which arises due to any charged groups on the surface of the particle and the
attractive van der Waals term.
to an ‘electric double layer’ consisting essentially of two distinct regions;
an inner region of adsorbed ions called the Stern layer and a more diffuse
region. When two such diffuse layers overlap, a repulsive interaction is
3
introduced. For typical ionic strengths, e.g. 10 3 moldm , the thickness of
the double layer is about 10nm. If the ionic strength is substantially higher,
the double-layer interaction is sufficiently reduced and it can no longer
provide stabilisation against the van der Waals driven aggregation. In con-
trast to the van der Waals interaction which falls off reciprocally with dis-
tance, the electrostatic repulsion falls off exponentially with distance.
Consequently, the van der Waals interaction dominates at small and large
distances, whilst the double-layer interaction dominates at intermediate
distances. The interparticle interaction has the form shown in Figure 6.3.