Page 136 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 136
3 COMMON APPARATUS AN0 BASIC TECHNIQUES
In some circumstances it rnay be considered preferable to prepare the standard
solution by making use of one of the concentrated volumetric solutions supplied
in sealed ampoules which only require dilution in a graduated flask to produce
a standard solution.
Solutions which are comparatively stable and unaffected by exposure to air
rnay be stored in 1 litre or 2.5 litre bottles; for work requiring the highest
accuracy, the bottles should be Pyrex, or other resistance glass, and fitted with
ground-glass stoppers: the solvent action of the solution being thus considerably
reduced. It is however necessary to use a rubber bung instead of a glass stopper
for alkaline solutions, and in many instances a polythene container rnay well
replace glass vessels. It should be noted, however, that for some solutions as,
for example, iodine and silver nitrate, glass containers only rnay be used, and
in both these cases the bottle should be made of dark (brown) glass: solutions
of EDTA (Section 10.49) are best stored in polythene containers.
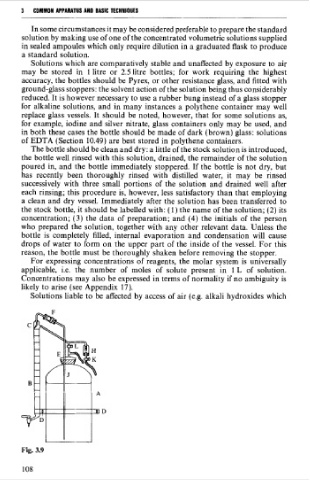
The bottle should be clean and dry: a little of the stock solution is introduced,
the bottle well rinsed with this solution, drained, the remainder of the solution
poured in, and the bottle immediately stoppered. If the bottle is not dry, but
has recently been thoroughly rinsed with distilled water, it rnay be rinsed
successively with three small portions of the solution and drained well after
each rinsing; this procedure is, however, less satisfactory than that employing
a clean and dry vessel. Immediately after the solution has been transferred to
the stock bottle, it should be labelled with: (1) the name of the solution; (2) its
concentration; (3) the data of preparation; and (4) the initials of the person
who prepared the solution, together with any other relevant data. Unless the
bottle is completely filled, interna1 evaporation and condensation will cause
drops of water to form on the upper part of the inside of the vessel. For this
reason, the bottle must be thoroughly shaken before removing the stopper.
For expressing concentrations of reagents, the molar system is universally
applicable, i.e. the number of moles of solute present in 1 L of solution.
Concentrations rnay also be expressed in terms of normality if no ambiguity is
likely to arise (see Appendix 17).
Solutions liable to be affected by access of air (e.g. alkali hydroxides which
Fig. 3.9