Page 137 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 137
PREPARATION OF THE SUBSTANCE FOR ANALYSIS 3.29
absorb carbon dioxide; iron(I1) and titanium(II1) which are oxidised) may be
stored in the apparatus shown diagrammatically in Fig. 3.9. A is a large storage
bottle of 10-15 litres capacity. B is a 50 mL burette provided with an automatic
filling device at C (the point of the drawn-out tube is adjusted to be exactly at
the zero mark of the burette), D is the burette-bottle clamp, E is a two-holed
ground-glass joint, F is a ground-glass tension joint, a rubber tube is connected
to a hydrogen cylinder and to the 1-joint below L, H is a Bunsen valve, and J
is hydrogen. The burette is filled by closing tap K and passing hydrogen through
the rubber tube attached to the 1-piece (below tap L) with tap L closed; taps L
and K are opened, and the excess of liquid allowed to siphon back.
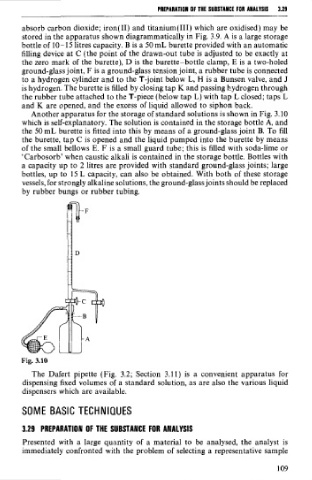
Another apparatus for the storage of standard solutions is shown in Fig. 3.10
which is self-explanatory. The solution is contained in the storage bottle A, and
the 50 mL burette is fitted into this by means of a ground-glass joint B. To fil1
the burette, tap C is opened and the liquid pumped into the burette by means
of the small bellows E. F is a small guard tube; this is filled with soda-lime or
'Carbosorb' when caustic alkali is contained in the storage bottle. Bottles with
a capacity up to 2 litres are provided with standard ground-glass joints; large
bottles, up to 15 L capacity, can also be obtained. With both of these storage
vessels, for strongly alkaline solutions, the ground-glass joints should be replaced
by rubber bungs or rubber tubing.
Fig. 3.10
The Dafert pipette (Fig. 3.2; Section 3.11) is a convenient apparatus for
dispensing fixed volumes of a standard solution, as are also the various liquid
dispensers which are available.
SOME BASIC TECHNIQUES
3.29 PREPARATION OF THE SUBSTANCE FOR ANALYSIS
Presented with a large quantity of a material to be analysed, the analyst is
immediately confronted with the problem of selecting a representative sample