Page 194 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 194
6 SOLVENT EXTRACTION
and the percentage of solute extracted, E, is given by
log E - log(100 - E) = log D
The distribution of the metal in a given system of the above type is a function
of the pH alone. The equation represents a family of sigmoid curves when E is
plotted against pH, with the position of each along the pH axis depending only
on the magnitude of K* and the slope of each uniquely depending upon n.
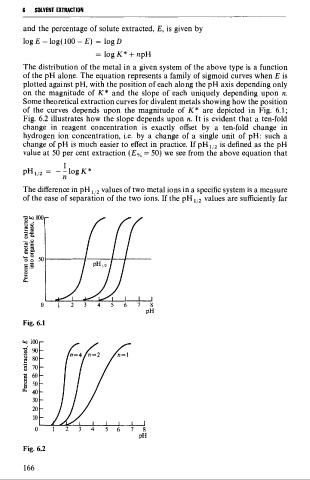
Some theoretical extraction curves for divalent metals showing how the position
of the curves depends upon the magnitude of K* are depicted in Fig. 6.1;
Fig. 6.2 illustrates how the slope depends upon n. It is evident that a ten-fold
change in reagent concentration is exactly offset by a ten-fold change in
hydrogen ion concentration, i.e. by a change of a single unit of pH: such a
change of pH is much easier to effect in practice. If pHll2 is defined as the pH
value at 50 per cent extraction (E, = 50) we see from the above equation that
The difference in pH values of two metal ions in a specific system is a measure
of the ease of separation of the two ions. If the pH ,,, values are sufficiently far
Fig. 6.1
Fig. 6.2