Page 199 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 199
EXTRACTION REACENTS 6.6
Diphenylthiocarbazone (dithizone), C6HS-N=N-CS-NH-NHSC6Hs. The
compound is insoluble in water and dilute minera1 acids, and is readily soluble
in dilute aqueous ammonia. It is used in dilute solution in chloroform or carbon
tetrachloride. Dithizone is an important selective reagent for quantitative
determinations of metals: colorimetric (and, of course, spectrophotometric)
analyses are based upon the intense green colour of the reagent and the
contrasting colours of the metal dithizonates in organic solvents. The selectivity
is improved by the control of pH and the use of masking agents, such as cyanide,
thiocyanate, thiosulphate, and EDTA.
The use of dithizone in combination with various organic bases for synergistic
extraction has already been indicated (Section 6.4).
Sodium diethyldithiocarbamate, {(C2HS),N-CS-S)-Na'. This reagent is
generally used as a 2 per cent aqueous solution; it decomposes rapidly in
solutions of low pH. It is an effective extraction reagent for over 20 metals into
various organic solvents, such as chloroform, carbon tetrachloride, and ethanol.
The selectivity is enhanced by the control of pH and the addition of masking
agents.
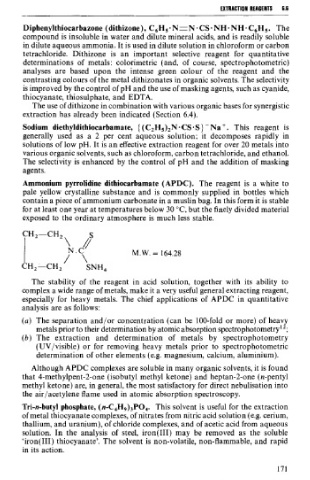
Ammonium pyrrolidine dithiocarbamate (APDC). The reagent is a white to
pale yellow crystalline substance and is commonly supplied in bottles which
contain a piece of ammonium carbonate in a muslin bag. In this form it is stable
for at least one year at temperatures below 30 OC, but the finely divided material
exposed to the ordinary atmosphere is much less stable.
The stability of the reagent in acid solution, together with its ability to
complex a wide range of metals, make it a very useful general extracting reagent,
especially for heavy metals. The chief applications of APDC in quantitative
analysis are as follows:
(a) The separation and/or concentration (can be 100-fold or more) of heavy
metals prior to their determination by atomic absorption spectrophotometry12;
(b) The extraction and determination of metals by spectrophotometry
(UV/visible) or for removing heavy metals prior to spectrophotometric
determination of other elements (e.g. magnesium, calcium, aluminium).
Although APDC complexes are soluble in many organic solvents, it is found
that 4-methylpent-Zone (isobutyl methyl ketone) and heptan-Zone (n-pentyl
methyl ketone) are, in general, the most satisfactory for direct nebulisation into
the airlacetylene flame used in atomic absorption spectroscopy.
Tri-n-butyl phosphate, (n-C4H,),P04. This solvent is useful for the extraction
of metal thiocyanate complexes, of nitrates from nitric acid solution (e.g. cerium,
thallium, and uranium), of chloride complexes, and of acetic acid from aqueous
solution. In the analysis of steel, iron(II1) may be removed as the soluble
'iron(II1) thiocyanate'. The solvent is non-volatile, non-flammable, and rapid
in its action.