Page 203 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 203
DETERMINATION OF BORON USlNG FERROIN 6.9
trace metals in water has been described.15 Details of the use of automatic
analysers are best obtained by referring to the appropriate manufacturers'
manuals.
SOME APPLICATIONS
6.8 DETERMINATION OF BERYLLIUM AS THE ACETYLACETONE COMPLEX
Discussion. Beryllium forms an acetylacetone complex, which is soluble in
chloroform, and yields an absorption maximum at 295 nm. The excess of
acetylacetone in the chloroform solution may be removed by rapid washing
with O.1M-sodium hydroxide solution. It is advisable to treat the solution
containing up to 10pg of Be with up to 10 mL of 2 per cent EDTA solution:
the latter will mask up to 1 mg of Fe, Al, Cr, Zn, Cu, Pb, Ag, Ce, and U.
Procedure. Prepare a solution containing 10 pg of beryllium in 50 mL. (CARE:
Beryllium compounds are toxic.) Use beryllium sulphate, BeS0,,4H20. TO
50.0 mL of this solution contained in a beaker, add dilute hydrochloric acid
until the pH is 1.0, and then introduce 10.0 mL of 2 per cent EDTA solution.
Adjust the pH to 7 by the addition of 0.1M sodium hydroxide solution. Add
5.0 mL of 1 per cent aqueous acetylacetone and readjust the pH to 7-8. After
standing for 5 minutes, extract the colourless beryllium complex with three
10 mL portions of chloroform. Wash the chloroform extract rapidly with two
50mL portions of 0.1M sodium hydroxide in order to remove the excess of
acetylacetone. To determine the absorbance at 295 nm (in the ultraviolet region
of the spectrum) it may be necessary to dilute the extract with chloroform.
Measure the absorbance using 1.0 cm absorption cells against a blank.
Repeat the determination with a solution containing 100 pg of iron(II1) and
of aluminium ion: the absorbance is unaffected.
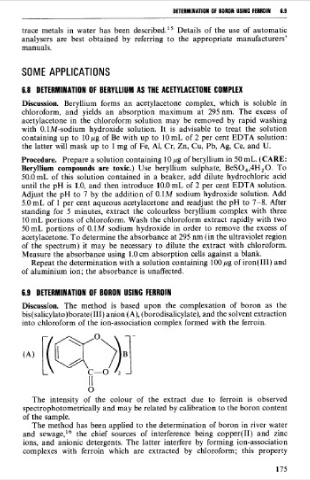
6.9 DETERMINATION OF BORON USlNG FERROIN
Discussion. The method is based upon the complexation of boron as the
bis(salicylato)borate(III) anion (A), (borodisalicylate), and the solvent extraction
into chloroform of the ion-association complex formed with the ferroin.
The intensity of the colour of the extract due to ferroin is observed
spectrophotometrically and may be related by calibration to the boron content
of the sample.
The method has been applied to the determination of boron in river water
and sewage,16 the chief sources of interference being copper(I1) and zinc
ions, and anionic detergents. The latter interfere by forming ion-association
complexes with ferroin which are extracted by chloroform; this property