Page 86 - Wastewater Solids Incineration Systems
P. 86
Combustion Technology 55
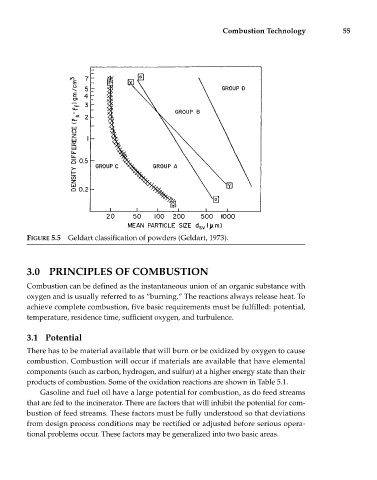
FIGURE 5.5 Geldart classification of powders (Geldart, 1973).
3.0 PRINCIPLES OF COMBUSTION
Combustion can be defined as the instantaneous union of an organic substance with
oxygen and is usually referred to as “burning.” The reactions always release heat. To
achieve complete combustion, five basic requirements must be fulfilled: potential,
temperature, residence time, sufficient oxygen, and turbulence.
3.1 Potential
There has to be material available that will burn or be oxidized by oxygen to cause
combustion. Combustion will occur if materials are available that have elemental
components (such as carbon, hydrogen, and sulfur) at a higher energy state than their
products of combustion. Some of the oxidation reactions are shown in Table 5.1.
Gasoline and fuel oil have a large potential for combustion, as do feed streams
that are fed to the incinerator. There are factors that will inhibit the potential for com-
bustion of feed streams. These factors must be fully understood so that deviations
from design process conditions may be rectified or adjusted before serious opera-
tional problems occur. These factors may be generalized into two basic areas.