Page 286 - Water and wastewater engineering
P. 286
LIME–SODA SOFTENING 7-3
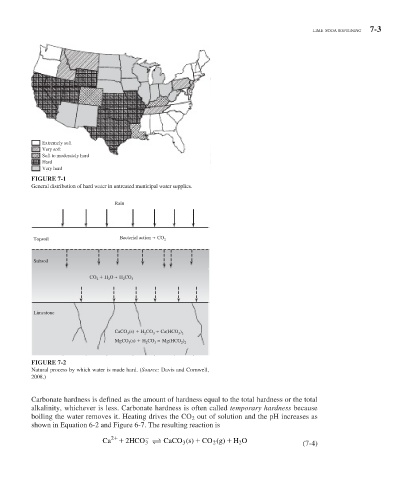
Extremely soft
Very soft
Soft to moderately hard
Hard
Very hard
FIGURE 7-1
General distribution of hard water in untreated municipal water supplies.
Rain
Topsoil Bacterial action CO 2
Subsoil
CO H O H CO
2 2 2 3
Limestone
(s) H CO Ca(HCO )
CaCO 3 2 3 3 2
MgCO (s) H CO 3 Mg(HCO )
2 2
2
3
FIGURE 7-2
Natural process by which water is made hard. (Source: Davis and Cornwell,
2008.)
Carbonate hardness is defined as the amount of hardness equal to the total hardness or the total
alkalinity, whichever is less. Carbonate hardness is often called temporary hardness because
boiling the water removes it. Heating drives the CO 2 out of solution and the pH increases as
shown in Equation 6-2 and Figure 6-7. The resulting reaction is
2
Ca 2HCO 3 CaCO s () CO g ( ) H O
3 2 2 (7-4)