Page 287 - Water and wastewater engineering
P. 287
7-4 WATER AND WASTEWATER ENGINEERING
Noncarbonate hardness is defined as the total hardness in excess of the alkalinity. If the alkalin-
ity is equal to or greater than the total hardness, then there is no noncarbonate hardness. Noncarbon-
ate hardness is called permanent hardness because it is not removed when water is heated.
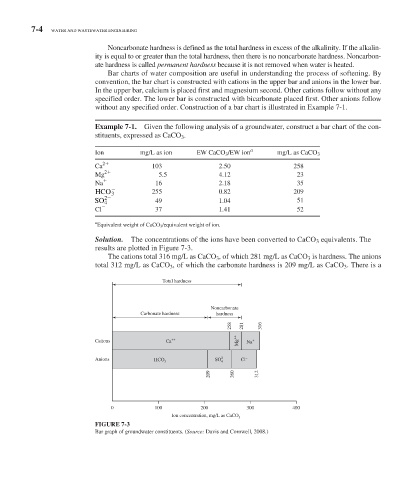
Bar charts of water composition are useful in understanding the process of softening. By
convention, the bar chart is constructed with cations in the upper bar and anions in the lower bar.
In the upper bar, calcium is placed first and magnesium second. Other cations follow without any
specified order. The lower bar is constructed with bicarbonate placed first. Other anions follow
without any specified order. Construction of a bar chart is illustrated in Example 7-1.
Example 7-1. Given the following analysis of a groundwater, construct a bar chart of the con-
stituents, expressed as CaCO 3 .
Ion mg/L as ion EW CaCO 3 /EW ion a mg/L as CaCO 3
2
Ca 103 2.50 258
2
Mg 5.5 4.12 23
Na 16 2.18 35
HCO 3 255 0.82 209
2
SO 4 49 1.04 51
Cl 37 1.41 52
a
Equivalent weight of CaCO 3 /equivalent weight of ion.
Solution. The concentrations of the ions have been converted to CaCO 3 equivalents. The
results are plotted in Figure 7-3 .
The cations total 316 mg/L as CaCO 3 , of which 281 mg/L as CaCO 3 is hardness. The anions
total 312 mg/L as CaCO 3 , of which the carbonate hardness is 209 mg/L as CaCO 3 . There is a
Total hardness
Noncarbonate
Carbonate hardness hardness
258 281 316
Mg ++ Na
Cations ++ +
Ca
HCO –
3
Anions HCO – SO 2– Cl –
3 4
209 260 312
0 100 200 300 400
Ion concentration, mg/L as CaCO
3
FIGURE 7-3
Bar graph of groundwater constituents. (Source: Davis and Cornwell, 2008.)