Page 81 - Mechanical Behavior of Materials
P. 81
82 Chapter 3 A Survey of Engineering Materials
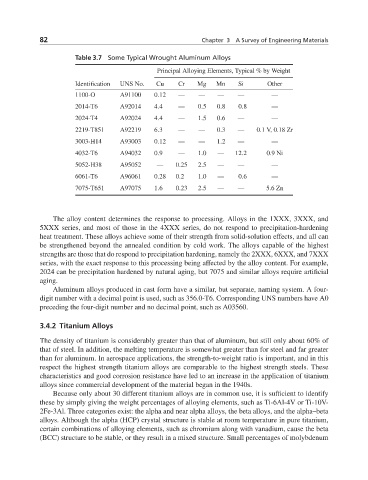
Table 3.7 Some Typical Wrought Aluminum Alloys
Principal Alloying Elements, Typical % by Weight
Identification UNS No. Cu Cr Mg Mn Si Other
1100-O A91100 0.12 — — — — —
2014-T6 A92014 4.4 — 0.5 0.8 0.8 —
2024-T4 A92024 4.4 — 1.5 0.6 — —
2219-T851 A92219 6.3 — — 0.3 — 0.1 V, 0.18 Zr
3003-H14 A93003 0.12 — — 1.2 — —
4032-T6 A94032 0.9 — 1.0 — 12.2 0.9 Ni
5052-H38 A95052 — 0.25 2.5 — — —
6061-T6 A96061 0.28 0.2 1.0 — 0.6 —
7075-T651 A97075 1.6 0.23 2.5 — — 5.6 Zn
The alloy content determines the response to processing. Alloys in the 1XXX, 3XXX, and
5XXX series, and most of those in the 4XXX series, do not respond to precipitation-hardening
heat treatment. These alloys achieve some of their strength from solid-solution effects, and all can
be strengthened beyond the annealed condition by cold work. The alloys capable of the highest
strengths are those that do respond to precipitation hardening, namely the 2XXX, 6XXX, and 7XXX
series, with the exact response to this processing being affected by the alloy content. For example,
2024 can be precipitation hardened by natural aging, but 7075 and similar alloys require artificial
aging.
Aluminum alloys produced in cast form have a similar, but separate, naming system. A four-
digit number with a decimal point is used, such as 356.0-T6. Corresponding UNS numbers have A0
preceding the four-digit number and no decimal point, such as A03560.
3.4.2 Titanium Alloys
The density of titanium is considerably greater than that of aluminum, but still only about 60% of
that of steel. In addition, the melting temperature is somewhat greater than for steel and far greater
than for aluminum. In aerospace applications, the strength-to-weight ratio is important, and in this
respect the highest strength titanium alloys are comparable to the highest strength steels. These
characteristics and good corrosion resistance have led to an increase in the application of titanium
alloys since commercial development of the material began in the 1940s.
Because only about 30 different titanium alloys are in common use, it is sufficient to identify
these by simply giving the weight percentages of alloying elements, such as Ti-6Al-4V or Ti-10V-
2Fe-3Al. Three categories exist: the alpha and near alpha alloys, the beta alloys, and the alpha–beta
alloys. Although the alpha (HCP) crystal structure is stable at room temperature in pure titanium,
certain combinations of alloying elements, such as chromium along with vanadium, cause the beta
(BCC) structure to be stable, or they result in a mixed structure. Small percentages of molybdenum