Page 246 - Materials Chemistry, Second Edition
P. 246
Technologies for Treatment of Heavy Metal–Contaminated Groundwater 227
Reduction in Decrease in
photosynthesis water potential
Inhibition of Heavy metal Protein
growth toxicity in oxidation
plants
Enzyme Nucleic acid
inhibition Cell death damage
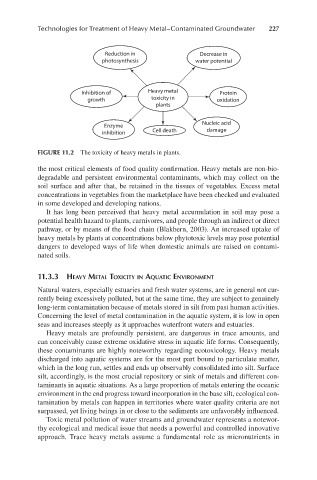
FIGURE 11.2 The toxicity of heavy metals in plants.
the most critical elements of food quality confirmation. Heavy metals are non-bio-
degradable and persistent environmental contaminants, which may collect on the
soil surface and after that, be retained in the tissues of vegetables. Excess metal
concentrations in vegetables from the marketplace have been checked and evaluated
in some developed and developing nations.
It has long been perceived that heavy metal accumulation in soil may pose a
potential health hazard to plants, carnivores, and people through an indirect or direct
pathway, or by means of the food chain (Blakbern, 2003). An increased uptake of
heavy metals by plants at concentrations below phytotoxic levels may pose potential
dangers to developed ways of life when domestic animals are raised on contami-
nated soils.
11.3.3 Heavy MeTal ToxiciTy in aQuaTic environMenT
Natural waters, especially estuaries and fresh water systems, are in general not cur-
rently being excessively polluted, but at the same time, they are subject to genuinely
long-term contamination because of metals stored in silt from past human activities.
Concerning the level of metal contamination in the aquatic system, it is low in open
seas and increases steeply as it approaches waterfront waters and estuaries.
Heavy metals are profoundly persistent, are dangerous in trace amounts, and
can conceivably cause extreme oxidative stress in aquatic life forms. Consequently,
these contaminants are highly noteworthy regarding ecotoxicology. Heavy metals
discharged into aquatic systems are for the most part bound to particulate matter,
which in the long run, settles and ends up observably consolidated into silt. Surface
silt, accordingly, is the most crucial repository or sink of metals and different con-
taminants in aquatic situations. As a large proportion of metals entering the oceanic
environment in the end progress toward incorporation in the base silt, ecological con-
tamination by metals can happen in territories where water quality criteria are not
surpassed, yet living beings in or close to the sediments are unfavorably influenced.
Toxic metal pollution of water streams and groundwater represents a notewor-
thy ecological and medical issue that needs a powerful and controlled innovative
approach. Trace heavy metals assume a fundamental role as micronutrients in