Page 300 - Materials Chemistry, Second Edition
P. 300
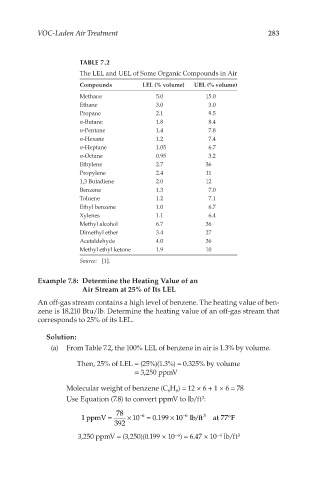
VOC-Laden Air Treatment 283
TABLE 7.2
The LEL and UEL of Some Organic Compounds in Air
Compounds LEL (% volume) UEL (% volume)
Methane 5.0 15.0
Ethane 3.0 3.0
Propane 2.1 9.5
n-Butane 1.8 8.4
n-Pentane 1.4 7.8
n-Hexane 1.2 7.4
n-Heptane 1.05 6.7
n-Octane 0.95 3.2
Ethylene 2.7 36
Propylene 2.4 11
1,3 Butadiene 2.0 12
Benzene 1.3 7.0
Toluene 1.2 7.1
Ethyl benzene 1.0 6.7
Xylenes 1.1 6.4
Methyl alcohol 6.7 36
Dimethyl ether 3.4 27
Acetaldehyde 4.0 36
Methyl ethyl ketone 1.9 10
Source: [1].
Example 7.8: Determine the Heating Value of an
Air Stream at 25% of Its LEL
An off-gas stream contains a high level of benzene. The heating value of ben-
zene is 18,210 Btu/lb. Determine the heating value of an off-gas stream that
corresponds to 25% of its LEL.
Solution:
(a) From Table 7.2, the 100% LEL of benzene in air is 1.3% by volume.
Then, 25% of LEL = (25%)(1.3%) = 0.325% by volume
= 3,250 ppmV
Molecular weight of benzene (C H ) = 12 × 6 + 1 × 6 = 78
6
6
3
Use Equation (7.8) to convert ppmV to lb/ft :
78
°
×
−6
1ppmV = × 10 −6 = 0.199 10 lb/ft 3 at77F
392
3,250 ppmV = (3,250)(0.199 × 10 ) = 6.47 × 10 lb/ft 3
−4
−6