Page 307 - Materials Chemistry, Second Edition
P. 307
290 Practical Design Calculations for Groundwater and Soil Remediation
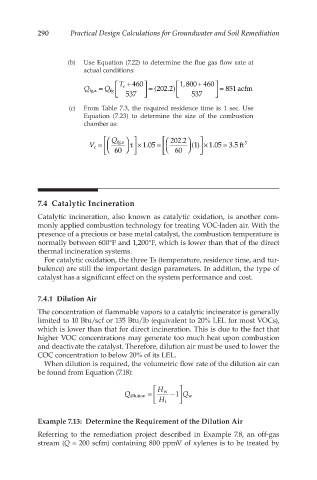
(b) Use Equation (7.22) to determine the flue gas flow rate at
actual conditions:
T c + 460 1,800 + 460
Q fg,a = Q fg 537 = (202.2) 537 = 851 acfm
(c) From Table 7.3, the required residence time is 1 sec. Use
Equation (7.23) to determine the size of the combustion
chamber as:
Q fg,a 202.2
V c = 1.05 = (1) × 1.05 = 3.5 ft 3
τ ×
60 60
7.4 Catalytic Incineration
Catalytic incineration, also known as catalytic oxidation, is another com-
monly applied combustion technology for treating VOC-laden air. With the
presence of a precious or base metal catalyst, the combustion temperature is
normally between 600°F and 1,200°F, which is lower than that of the direct
thermal incineration systems.
For catalytic oxidation, the three Ts (temperature, residence time, and tur-
bulence) are still the important design parameters. In addition, the type of
catalyst has a significant effect on the system performance and cost.
7.4.1 Dilution Air
The concentration of flammable vapors to a catalytic incinerator is generally
limited to 10 Btu/scf or 135 Btu/lb (equivalent to 20% LEL for most VOCs),
which is lower than that for direct incineration. This is due to the fact that
higher VOC concentrations may generate too much heat upon combustion
and deactivate the catalyst. Therefore, dilution air must be used to lower the
COC concentration to below 20% of its LEL.
When dilution is required, the volumetric flow rate of the dilution air can
be found from Equation (7.18):
H w
Q dilution = − 1 Q w
H i
Example 7.13: Determine the Requirement of the Dilution Air
Referring to the remediation project described in Example 7.8, an off-gas
stream (Q = 200 scfm) containing 800 ppmV of xylenes is to be treated by