Page 23 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 23
CHAPTER 1 History of Low-Salinity and Smart Waterflood 15
with 5761 ppm. Brines of key ions and smart water have 4.4 14
2þ 2þ 2
all ions of Ca ,Mg , and SO 4 as well as NaCl. The 4.2 13
brine of key ions has 3.5 times higher concentration of 12
divalent ions and lower concentration of NaCl than 4.0
smart water brine. The z-PALS estimates the z-potential 3.8 11
of binary systems of brine/oil droplet and brine/calcite. 10
Generally, the opposite charge of the binary systems 3.6 9
results in the electrostatic attraction between the two in- 3.4 8
terfaces of brine/oil droplet and brine/calcite. The
attraction makes the oil droplet adheres to rock surface. pCa 3.2 7 μ Ca 2+ Adsorbed ( moles/gm CaCO 3 )
When both interfaces have negative charge, an electro- 3.0 6
static repulsion occurs and a brine film stabilizes be- 5
2.8
tween the interfaces modifying wettability toward 4
water-wetness. The experiments report that the binary 2.6
3
system of brine/oil droplet shows the negative z-poten- 2.4
tial regardless of brine type. In the binary system of 2
brine/calcite, the cases of NaCl, Na 2 SO 4 , and smart 2.2 1
water show the negative z-potential, but the other 2.0 0
show the positive z-potential. The increasing positive 0 2 4 6 8 10 12 14 16 18 20
z-potential for key ions brine is attributed to the higher Zeta – potential (mV)
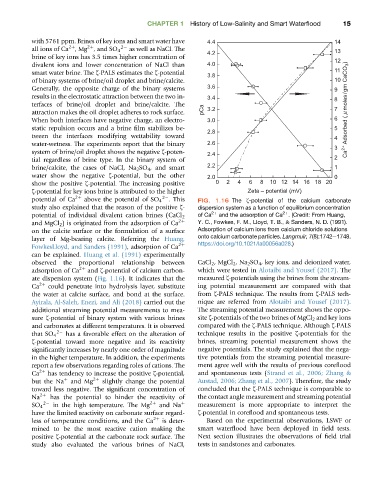
potential of Ca 2þ above the potential of SO 4 2 . This FIG. 1.16 The z-potential of the calcium carbonate
study also explained that the reason of the positive z- dispersion system as a function of equilibrium concentration
2þ
of Ca 2þ and the adsorption of Ca . (Credit: From Huang,
potential of individual divalent cation brines (CaCl 2
and MgCl 2 ) is originated from the adsorption of Ca 2þ Y. C., Fowkes, F. M., Lloyd, T. B., & Sanders, N. D. (1991).
on the calcite surface or the formulation of a surface Adsorption of calcium ions from calcium chloride solutions
layer of Mg-bearing calcite. Referring the Huang, onto calcium carbonate particles. Langmuir, 7(8):1742e1748.
FowkesLloyd, and Sanders (1991), adsorption of Ca 2þ https://doi.org/10.1021/la00056a028.)
can be explained. Huang et al. (1991) experimentally
observed the proportional relationship between CaCl 2 , MgCl 2 ,Na 2 SO 4 , key ions, and deionized water,
adsorption of Ca 2þ and z-potential of calcium carbon- which were tested in Alotaibi and Yousef (2017). The
ate dispersion system (Fig. 1.16). It indicates that the measured z-potentials using the brines from the stream-
Ca 2þ could penetrate into hydrolysis layer, substitute ing potential measurement are compared with that
the water at calcite surface, and bond at the surface. from z-PALS technique. The results from z-PALS tech-
Ayirala, Al-Saleh, Enezi, and Ali (2018) carried out the nique are referred from Alotaibi and Yousef (2017).
additional streaming potential measurements to mea- The streaming potential measurement shows the oppo-
sure z-potential of binary system with various brines site z-potentials of the two brines of MgCl 2 and key ions
and carbonates at different temperatures. It is observed compared with the z-PALS technique. Although z-PALS
2 has a favorable effect on the alteration of technique results in the positive z-potentials for the
that SO 4
z-potential toward more negative and its reactivity brines, streaming potential measurement shows the
significantly increases by nearly one order of magnitude negative potentials. The study explained that the nega-
in the higher temperature. In addition, the experiments tive potentials from the streaming potential measure-
report a few observations regarding roles of cations. The ment agree well with the results of previous coreflood
Ca 2þ has tendency to increase the positive z-potential, and spontaneous tests (Strand et al., 2006; Zhang &
but the Na þ and Mg 2þ slightly change the potential Austad, 2006; Zhang et al., 2007). Therefore, the study
toward less negative. The significant concentration of concluded that the z-PALS technique is comparable to
Na 2þ has the potential to hinder the reactivity of the contact angle measurement and streaming potential
2 2þ þ measurement is more appropriate to interpret the
SO 4 in the high temperature. The Mg and Na
have the limited reactivity on carbonate surface regard- z-potential in coreflood and spontaneous tests.
less of temperature conditions, and the Ca 2þ is deter- Based on the experimental observations, LSWF or
mined to be the most reactive cation making the smart waterflood have been deployed in field tests.
positive z-potential at the carbonate rock surface. The Next section illustrates the observations of field trial
study also evaluated the various brines of NaCl, tests in sandstones and carbonates.