Page 191 - A Practical Companion to Reservoir Stimulation
P. 191
PRACTICAL COMPANION TO RESERVOIR STIMULATION
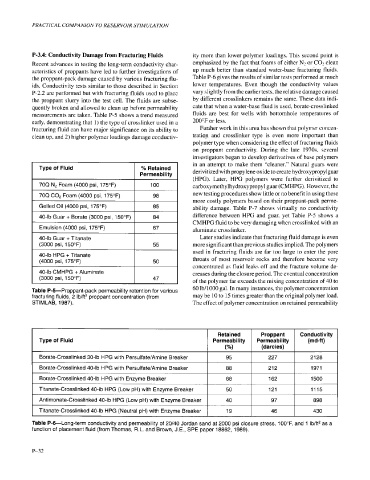
P-3.4: Conductivity Damage from Fracturing Fluids ity more than lower polymer loadings. This second point is
Recent advances in testing the long-term conductivity char- emphasized by the fact that foams of either Nz or CO- c 1 ean
acteristics of proppants have led to further investigations of up much better than standard water-base fracturing fluids.
the proppant-pack damage caused by various fracturing flu- Table P-6 gives the results of similar tests performed at much
ids. Conductivity tests similar to those described in Section lower temperatures. Even though the conductivity values
P-2.2 are performed but with fracturing fluids used to place vary slightly from the earlier tests, the relative damage caused
the proppant slurry into the test cell. The fluids are subse- by different crosslinkers remains the same. These data indi-
quently broken and allowed to clean up before permeability cate that when a water-base fluid is used, borate-crosslinked
measurements are taken. Table P-5 shows a trend measured fluids are best for wells with bottomhole temperatures of
early, demonstrating that 1) the type of crosslinker used in a 200°F or less.
fracturing fluid can have major significance on its ability to Further work in this area has shown that polymer concen-
clean up, and 2) higher polymer loadings damage conductiv- tration and crosslinker type is even more important than
polymer type when considering the effect of fracturing fluids
on proppant conductivity. During the late 1970~ several
investigators began to develop derivatives of base polymers
in an attempt to make them "cleaner." Natural guars were
Type of Fluid YO Retained derivitized with propylene oxide to create hydroxypropylguar
Permeability
(HPG). Later, HPG polymers were further derivitized to
70Q N2 Foam (4000 psi, 175°F) 100 carboxymethylhydroxypropyl guar (CMHPG). However, the
new testing procedures show little or no benefit in using these
70Q C02 Foam (4000 psi, 175°F) 98
more costly polymers based on their proppant-pack perme-
Gelled Oil (4000 psi, 175°F) 85 ability damage. Table P-7 shows virtually no conductivity
40-lb Guar + Borate (3000 psi, 150°F) difference between HPG and guar, yet Table P-5 shows a
CMHPG fluid to be very damaging when crosslinked with an
Emulsion (4000 psi, 175°F) aluminate crosslinker.
40-lb Guar + Titanate Later studies indicate that fracturing fluid damage is even
(3000 psi, 150°F) more significant than previous studies implied. The polymers
used in fracturing fluids are far too large to enter the pore
40-lb HPG + Titanate
(4000 psi, 175°F) throats of most reservoir rocks and therefore become very
concentrated as fluid leaks off and the fracture volume de-
40-lb CMHPG + Aluminate creases during the closure period. The eventual concentration
(3000 psi, 150°F) of the polymer far exceeds the mixing concentration of 40 to
Table Pd-Proppant-pack permeability retention for various 60 lb/1000 gal. In many instances, the polymer concentration
fracturing fluids, 2 Ib/ft2 proppant concentration (from may be 10 to 15 times greater than the original polymer load.
STIMLAB, 1987). The effect of polymer concentration on retained permeability
Retained Proppant Conductivity
Type of Fluid Permeability Permeability (md-ft)
("/.I (darcies)
Borate-Crosslinked 30-lb HPG with Persulfate/Amine Breaker 95 227 2128
I Borate-Crosslinked 40-lb HPG with Persulfate/Amine Breaker I 88 I 212 I 1971
Borate-Crosslinked 40-lb HPG with Enzyme Breaker 68 162 1500
Titanate-Crosslinked 40-lb HPG (Low pH) with Enzyme Breaker 50 121 1115
Antimonate-Crosslinked 40-lb HPG (Low pH) with Enzyme Breaker 40 97 898
Titanate-Crosslinked 40-lb HPG (Neutral pH) with Enzyme Breaker 19 46 430
Table Pd-Long-term conductivity and permeability of 20/40 Jordan sand at 2000 psi closure stress, lOO"F, and 1 Ib/ft2 as a
function of placement fluid (from Thomas, R.L. and Brown, J.E., SPE paper 18862, 1989).
P-32