Page 194 - A Practical Companion to Reservoir Stimulation
P. 194
PRACTICAL CONSIDERATIONS FOR FRACTURE TREATMENT DESIGN
P-3.5: Viscosity Reduction from Breakers
Breakers are added to fracturing fluids for two reasons. First, 100
the viscosity of the fluid must be reduced so that the fluid can
be cleaned up quickly following a treatment. Second, as 90
demonstrated in Section P-3.4, the breaker should also de- 80
grade the fluid and thus reduce proppant-conductivity dam-
age. All common breakers perform both these tasks by attack- 70
ing the backbone of the polymer and reducing its size. As the
molecular weight of the polymers decreases, so does the 60
fluid's viscosity. 50
Several systems are currently used to break water-base
fracturing fluids. Table P-8 shows the concentration of con- 40
ventional breaker systems needed to break linear fluids when 30
the well can be shut in for 24 hr. Enzyme breakys can be
effective over a relatively wide temperature range (70°F to 20
150°F) but are limited to a pH range between 3.5 and 8. The
optimum pH for most enzyme breakers is 5. Oxidizing breakers 10
are used in applications where the fluid is exposed to 0
bottomhole temperatures between 125°F and 225°F. These Persulfate/ Enzyme
breakers can be expanded into lower temperature applications Amine Breaker
(60°F to 125°F) if an amine is concurrently added to catalyze Breaker
the reaction. These breakers are effective over a wide pH
range (3 to 14) and demonstrate superior breaking properties
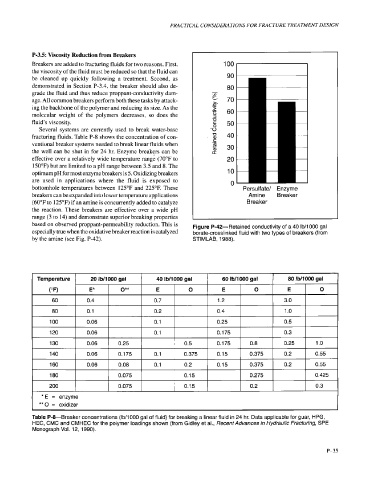
based on observed proppant-permeability reduction. This is Figure P-42-Retained conductivity of a 40 Ib/lOOO gal
especially true when the oxidative breaker reaction is catalyzed borate-crosslinked fluid with two types of breakers (from
by the amine (see Fig. P-42). STIMLAB, 1988).
1 I I I I I I I
I 140 I 0.06 I 0.175 I 0.1 I 0.375 I 0.15 I 0.375 I 0.2 I 0.55 I
160 0.06 0.08 0.1 0.2 0.15 0.375 0.2 0.55
180 0.075 0.1 5 0.275 0.425
200 0.075 0.15 0.2 0.3
Table P-8-Breaker concentrations (Ib/l000 gal of fluid) for breaking a linear fluid in 24 hr. Data applicable for guar, HPG,
HEC, CMC and CMHEC for the polymer loadings shown (from Gidley et al., RecentAdvances in Hydraulic Fracturing, SPE
Monograph Vol. 12, 1990).
P-35