Page 232 - A Practical Companion to Reservoir Stimulation
P. 232
PRACTICAL CONSIDERATIONS FOR FRACTURE TREATMENT DESIGN
P-8
of the gelling agent and may interfere with the crosslinking
Location Quality Assurance chemistry of many fluids. Levels of bicarbonate can be
changed by altering the pH of the fluid (Fig. P-73). There is
A significant effort is made in the design process to determine always a balance between the different C02-related ions in
an optimum fracturing treatment. An equal effort toward solution with the various amounts of carbonate, bicarbonate
quality assurance ensures that the treatment is executed as and C02 gas. An accurate understanding of bicarbonates
designed. Simple quality assurance steps can greatly increase must also be accompanied with a pH measurement. When
the odds of success for a hydraulic fracturing treatment. bicarbonates are measured on fluids that are to be crosslinked,
P-8.1: Fluids the fluid must be tested at the full pH range between hydra-
The fracturing fluid must hydraulically create the fracture tion and cmsslinking.
The iron content should be less than 25 mglliter. Excess
and transport the proppant to make an effective conduit for iron accelerates the development of free radicals from the
production. If the fluid does not have the rheological or polymer molecules. These free radicals increase the degrada-
leakoff properties used in the design process, the ultimate tion process of the polymer, especially at temperatures above
well performance will suffer. Great care must be taken to 200°F. If an oxidizer has been premixed in metal frac tanks,
ensure that the fluid performs as designed. Several simple the tanks undergo an oxidation reduction reaction. Part of the
tests performed on location can detect problems before oxidizing breaker spends itself in this reaction, resulting in a
pumping operations begin. At this time, steps can be taken to lower total breaker amount that produces unpredictable break
correct any deficiencies, ensuring that the rheological prop- times once the fracturing fluid is pumped.
erties of the fluid are adequate. Additives remaining from previous treatments in the frac
P-8.1.1: Initial Water Quality tank can be detrimental to fracturing fluid quality. Surfactants
Water used for the fracturing treatment must meet certain can create foaming problems during the mixing procedure. If
the foam problem becomes severe, circulation rates will be
standards before the fracturing fluids are prepared. Meeting reduced as the mixing pumps lose prime, leading to excessive
these standards greatly increases the odds that the fluids will additions of gelling material and lumping of the base gel. The
perform as designed. The temperature of the mix water presence of reducing agents can interfere with the crosslinking
should be between 50°F and 100°F for proper hydration. process or alter the break time. To test for reducing agents,
Certain polymers may hydrate at temperatures lower than add 2 drops of a 1 % potassium permanganate solution to 500
40°F if they are treated with buffer packages. However, ml of water having a pH between 5.5 and 7. A white precipi-
mixing fluids at low temperatures may result in low viscosi- tate forms if reducing agents are present; otherwise, the water
ties because some of the polymer may not completely hydrate turns light pink.
before settling out. At temperatures above 100°F, hydration
may occur so quickly that the individual powdered polymer P-8.1.2: Base Fluid Viscosity
crystals do not have sufficient time to disperse. If the polymer The ultimate viscosity of a crosslinked fluid depends on the
crystals are not well separated, the gel will develop lumps, or base viscosity of the linear gel. Figures P-74 through P-77
“fish eyes.” Low viscosities result from the unhydrated poly- can be used to check the base fluid viscosity for various
mer on the inside of the fish eye. polymer loadings. For Figs. P-74 and P-75, the viscosity must
The rate of hydration for polymers is very pH sensitive.
The time needed to hydrate a guar is seen in Fig. P-72. In
general, the pH of the water should be between 6 and 8 to
ensure proper hydration of a guar or derivitized guar polymer. Content
A pH greater than 8 retards the hydration development of
some polymers and can completely stop the hydration of 5,000
others. A pH less than 6 may result in lumping of the gel. The 10,000 1 1.64 I 2.10 I 1.56 I
viscosity of the gelled fluid may prematurely degrade if the
pH is lower than 4. 15,000 2.47 3.15 2.35
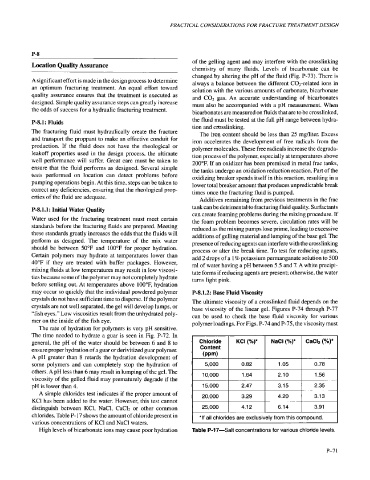
A simple chlorides test indicates if the proper amount of 20,000 3.29 4.20 3.13
KCl has been added to the water. However, this test cannot
distinguish between KCl, NaCl, CaC12 or other common 25,000 4.12 6.14 3.91
chlorides. Table P- 17 shows the amount of chloride present in
various concentrations of KCl and NaCl waters.
High levels of bicarbonate ions may cause poor hydration Table P-17-Salt concentrations for various chloride levels.
P-7 I